The Berkeleylactones, Antibiotic Macrolides from Fungal Coculture
- PMID: 28326781
- PMCID: PMC5467647
- DOI: 10.1021/acs.jnatprod.7b00133
The Berkeleylactones, Antibiotic Macrolides from Fungal Coculture
Erratum in
-
Correction to "The Berkeleylactones, Antibiotic Macrolides from Fungal Coculture".J Nat Prod. 2021 Dec 24;84(12):3169. doi: 10.1021/acs.jnatprod.1c01086. Epub 2021 Nov 23. J Nat Prod. 2021. PMID: 34813322 No abstract available.
Abstract
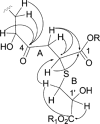
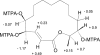
A carefully timed coculture fermentation of Penicillium fuscum and P. camembertii/clavigerum yielded eight new 16-membered-ring macrolides, berkeleylactones A-H (1, 4, 6-9, 12, 13), as well as the known antibiotic macrolide A26771B (5), patulin, and citrinin. There was no evidence of the production of the berkeleylactones or A26771B (5) by either fungus when grown as axenic cultures. The structures were deduced from analyses of spectral data, and the absolute configurations of compounds 1 and 9 were determined by single-crystal X-ray crystallography. Berkeleylactone A (1) exhibited the most potent antimicrobial activity of the macrolide series, with low micromolar activity (MIC = 1-2 μg/mL) against four MRSA strains, as well as Bacillus anthracis, Streptococcus pyogenes, Candida albicans, and Candida glabrata. Mode of action studies have shown that, unlike other macrolide antibiotics, berkeleylactone A (1) does not inhibit protein synthesis nor target the ribosome, which suggests a novel mode of action for its antibiotic activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Giddings L-A, Newman DJ. Bioactive Compounds from Terrestrial Extremophiles. In: Tiquia-Arashiro SM, Mormile M, editors. Springer Briefs in Microbiology; Extremophilic Bacteria. Springer; Heidelberg: 2015. pp. 6–61.pp. 65–73.
-
- Giddings L-A, Newman DJ. Bioactive Compounds from Marine Extremophiles. In: Tiquia-Arashiro SM, Mormile M, editors. Springer Briefs in Microbiology; Extremophilic Bacteria. Springer; Heidelberg: 2015. pp. 4–39.pp. 54–121.
-
-
Some of the previous reports of the isolation of secondary metabolites from Berkeley Pit fungi include:
- Stierle DB, Stierle AA, Patacini B, McIntyre K, Girtsman T, Bolstad E. J Nat Prod. 2011;74:2273–2277. - PMC - PubMed
- Stierle AA, Stierle DB, Girtsman T. J Nat Prod. 2012;75:344–350. - PMC - PubMed
- Stierle AA, Stierle DB. In: Studies in Natural Products Chemistry. Rahman Atta-Ur., editor. Vol. 39. Elsevier Science; Amsterdam: 2013. pp. 1–44.
- Stierle AA, Stierle DB, Mitman GG, Snyder S, Antczak C, Djaballah H. Nat Prod Commun. 2014;9:87–90. - PubMed
- Stierle AA, Stierle DB. Nat Prod Commun. 2014;9:1037–1044. - PMC - PubMed
-
-
- Thiery JP, Acioque H, Huang RYJ, Nieto MA. Cell. 2009;139:871–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

