Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn
- PMID: 28326935
- PMCID: PMC5315367
- DOI: 10.1177/1744806917693003
Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn
Abstract
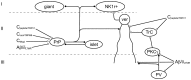
The spinal dorsal horn receives input from primary afferent axons, which terminate in a modality-specific fashion in different laminae. The incoming somatosensory information is processed through complex synaptic circuits involving excitatory and inhibitory interneurons, before being transmitted to the brain via projection neurons for conscious perception. The dorsal horn is important, firstly because changes in this region contribute to chronic pain states, and secondly because it contains potential targets for the development of new treatments for pain. However, at present, we have only a limited understanding of the neuronal circuitry within this region, and this is largely because of the difficulty in defining functional populations among the excitatory and inhibitory interneurons. The recent discovery of specific neurochemically defined interneuron populations, together with the development of molecular genetic techniques for altering neuronal function in vivo, are resulting in a dramatic improvement in our understanding of somatosensory processing at the spinal level.
Keywords: Itch; neuropeptide; pain; spinal cord.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

