Effect of Guizhifulingwan (Keishibukuryogan) on climacteric syndrome: study protocol for a randomized controlled pilot trial
- PMID: 28327172
- PMCID: PMC5361822
- DOI: 10.1186/s13063-017-1877-8
Effect of Guizhifulingwan (Keishibukuryogan) on climacteric syndrome: study protocol for a randomized controlled pilot trial
Abstract
Background: The aim of this study is to explore the efficacy of Guizhifulingwan (GFW) in the treatment of climacteric syndrome in women.
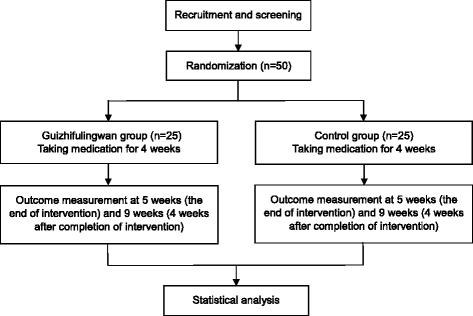
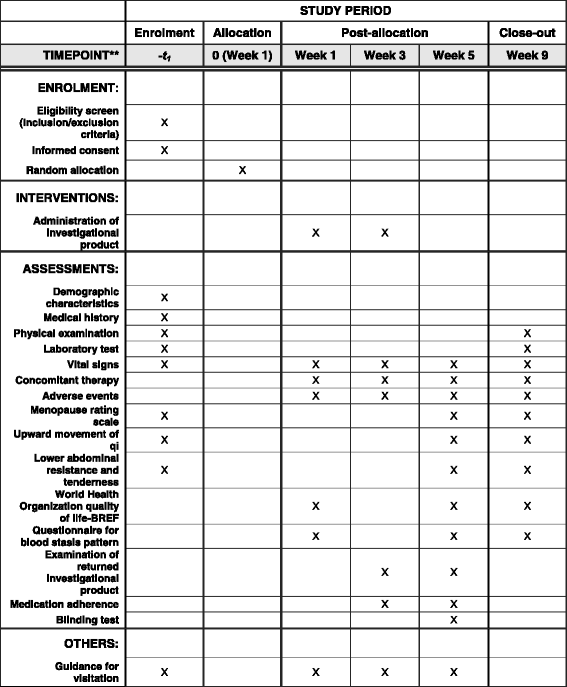
Methods/design: This is a single-center, randomized, placebo-controlled, parallel-group design pilot trial. Fifty participants with climacteric syndrome will be randomly allocated to the GFW or placebo group in a 1:1 ratio. The participants will be administered GFW or placebo granules three times a day for 4 weeks and will be followed up for a further 4 weeks. The primary outcome is the mean change in menopause rating scale score at 5 weeks after randomization. Secondary outcomes include the World Health Organization quality of life-BREF scores, degrees of upward movement of qi and lower abdominal resistance and tenderness, blood stasis pattern questionnaire scores, and results of blood tests including assays for lipid profile, high sensitivity C-reactive protein, follicle-stimulating hormone, and estradiol. The feasibility outcomes include recruitment and completion rates and adherence to medication.
Discussion: The results of this study will provide basic data for the design of a large-scale clinical trial for evaluating the efficacy of GFW in the treatment of climacteric syndrome in women.
Trial registration: Clinical Research Information Service (CRIS), Republic of Korea, KCT0002040 . Registered on 5 September 2016.
Keywords: Climacteric syndrome; Guizhifulingwan; Keishibukuryogan; Menopause; Randomized controlled trial.
Figures
Similar articles
-
Danggwijagyaksan for climacteric syndrome in peri- and postmenopausal women with a blood-deficiency dominant pattern: study protocol for a randomized, double-blind, placebo-controlled pilot trial.Trials. 2018 Jan 15;19(1):41. doi: 10.1186/s13063-018-2443-8. Trials. 2018. PMID: 29335018 Free PMC article.
-
Beneficial effect of Gyejibokryeong-hwan on climacteric syndrome with blood stasis pattern: A randomized, double-blinded, placebo-controlled clinical pilot trial.Integr Med Res. 2023 Jun;12(2):100951. doi: 10.1016/j.imr.2023.100951. Epub 2023 Apr 21. Integr Med Res. 2023. PMID: 37187679 Free PMC article.
-
The efficacy and safety of Danggui-Sayuk-Ga-Osuyu-Saenggang-tang on Korean patients with cold hypersensitivity in the hands: study protocol for a pilot, double-blind, randomized, placebo-controlled, parallel-group clinical trial.Trials. 2017 Jun 8;18(1):268. doi: 10.1186/s13063-017-2002-8. Trials. 2017. PMID: 28595610 Free PMC article. Clinical Trial.
-
Effects of herbal medicine (Danggwijagyaksan) for treating climacteric syndrome with a blood-deficiency-dominant pattern: A randomized, double-blind, placebo-controlled pilot trial.Integr Med Res. 2021 Sep;10(3):100715. doi: 10.1016/j.imr.2021.100715. Epub 2021 Jan 9. Integr Med Res. 2021. PMID: 33665100 Free PMC article.
-
Effects of dangguijakyaksan on lower-extremity blood circulation disturbances in climacteric and postmenopausal women: Study protocol for a randomized, double-blind, placebo-controlled pilot trial.Medicine (Baltimore). 2019 Sep;98(37):e17039. doi: 10.1097/MD.0000000000017039. Medicine (Baltimore). 2019. PMID: 31517823 Free PMC article.
Cited by
-
Exploring the Mechanism of Gyejibokryeong-hwan against Atherosclerosis Using Network Pharmacology and Molecular Docking.Plants (Basel). 2020 Dec 10;9(12):1750. doi: 10.3390/plants9121750. Plants (Basel). 2020. PMID: 33321972 Free PMC article.
-
Overcoming cisplatin resistance by targeting the MTDH-PTEN interaction in ovarian cancer with sera derived from rats exposed to Guizhi Fuling wan extract.BMC Complement Med Ther. 2020 Feb 17;20(1):57. doi: 10.1186/s12906-020-2825-9. BMC Complement Med Ther. 2020. PMID: 32066429 Free PMC article.
-
Exploring the mechanisms of Gui Zhi Fu Ling Wan on varicocele via network pharmacology and molecular docking.Andrologia. 2022 Dec;54(11):e14635. doi: 10.1111/and.14635. Epub 2022 Nov 13. Andrologia. 2022. PMID: 36372090 Free PMC article.
-
Efficacy and safety of Gyejibokryeong-hwan (GBH) in major depressive disorder: study protocol for multicentre randomised controlled trial.Trials. 2022 Jun 1;23(1):447. doi: 10.1186/s13063-022-06339-0. Trials. 2022. PMID: 35650612 Free PMC article.
-
Danggwijagyaksan for climacteric syndrome in peri- and postmenopausal women with a blood-deficiency dominant pattern: study protocol for a randomized, double-blind, placebo-controlled pilot trial.Trials. 2018 Jan 15;19(1):41. doi: 10.1186/s13063-018-2443-8. Trials. 2018. PMID: 29335018 Free PMC article.
References
-
- Collaborative Group on Epidemiological Studies Of Ovarian Cancer. Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, et al. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015;385:1835–42. doi: 10.1016/S0140-6736(14)61687-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials