Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis
- PMID: 28327548
- PMCID: PMC5520783
- DOI: 10.1038/npp.2017.51
Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis
Abstract
Cannabinoids, when co-administered with opioids, may enable reduced opioid doses without loss of analgesic efficacy (ie, an opioid-sparing effect). The aim of this study was to conduct a systematic review to determine the opioid-sparing potential of cannabinoids. Eligible studies included pre-clinical and clinical studies for which the outcome was either analgesia or opioid dose requirements. Clinical studies included controlled studies and case series. We searched Scopus, Cochrane Database of Systematic Reviews, Medline, and Embase. Nineteen pre-clinical and nine clinical studies met the search criteria. Seventeen of the 19 pre-clinical studies provided evidence of synergistic effects from opioid and cannabinoid co-administration. Our meta-analysis of pre-clinical studies indicated that the median effective dose (ED50) of morphine administered in combination with delta-9-tetrahydrocannabinol (delta-9-THC) is 3.6 times lower (95% confidence interval (CI) 1.95, 6.76; n=6) than the ED50 of morphine alone. In addition, the ED50 for codeine administered in combination with delta-9-THC was 9.5 times lower (95% CI 1.6, 57.5, n=2) than the ED50 of codeine alone. One case series (n=3) provided very-low-quality evidence of a reduction in opioid requirements with cannabinoid co-administration. Larger controlled clinical studies showed some clinical benefits of cannabinoids; however, opioid dose changes were rarely reported and mixed findings were observed for analgesia. In summary, pre-clinical studies provide robust evidence of the opioid-sparing effect of cannabinoids, whereas one of the nine clinical studies identified provided very-low-quality evidence of such an effect. Prospective high-quality-controlled clinical trials are required to determine the opioid-sparing effect of cannabinoids.
Figures
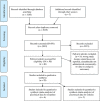
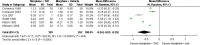


References
-
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL (2011). Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 90: 844–851. - PubMed
-
- Boehnke KF, Litinas E, Clauw DJ (2016). Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain 17: 739–744. - PubMed
-
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I et al (2015). The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain. Ann Intern Med 162: 276–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

