Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides
- PMID: 28327610
- PMCID: PMC5364439
- DOI: 10.1038/ncomms14913
Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides
Abstract
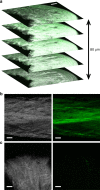
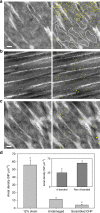
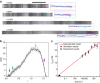
Mechanical injury to connective tissue causes changes in collagen structure and material behaviour, but the role and mechanisms of molecular damage have not been established. In the case of mechanical subfailure damage, no apparent macroscale damage can be detected, yet this damage initiates and potentiates in pathological processes. Here, we utilize collagen hybridizing peptide (CHP), which binds unfolded collagen by triple helix formation, to detect molecular level subfailure damage to collagen in mechanically stretched rat tail tendon fascicle. Our results directly reveal that collagen triple helix unfolding occurs during tensile loading of collagenous tissues and thus is an important damage mechanism. Steered molecular dynamics simulations suggest that a likely mechanism for triple helix unfolding is intermolecular shearing of collagen α-chains. Our results elucidate a probable molecular failure mechanism associated with subfailure injuries, and demonstrate the potential of CHP targeting for diagnosis, treatment and monitoring of tissue disease and injury.
Conflict of interest statement
Y.L. and S.M.Y. are cofounders of 3Helix which commercializes the CHP. All other authors have no competing interests to declare.
Figures




Similar articles
-
Tendons exhibit greater resistance to tissue and molecular-level damage with increasing strain rate during cyclic fatigue.Acta Biomater. 2021 Oct 15;134:435-442. doi: 10.1016/j.actbio.2021.07.045. Epub 2021 Jul 24. Acta Biomater. 2021. PMID: 34314889 Free PMC article.
-
Inflammatory cells do not decrease the ultimate tensile strength of intact tendons in vivo and in vitro: protective role of mechanical loading.J Appl Physiol (1985). 2007 Jan;102(1):11-7. doi: 10.1152/japplphysiol.00162.2006. Epub 2006 Aug 17. J Appl Physiol (1985). 2007. PMID: 16916923
-
Investigating mechanisms of tendon damage by measuring multi-scale recovery following tensile loading.Acta Biomater. 2017 Jul 15;57:363-372. doi: 10.1016/j.actbio.2017.04.011. Epub 2017 Apr 21. Acta Biomater. 2017. PMID: 28435080 Free PMC article.
-
Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading.Physiol Rev. 2004 Apr;84(2):649-98. doi: 10.1152/physrev.00031.2003. Physiol Rev. 2004. PMID: 15044685 Review.
-
From mechanical loading to collagen synthesis, structural changes and function in human tendon.Scand J Med Sci Sports. 2009 Aug;19(4):500-10. doi: 10.1111/j.1600-0838.2009.00986.x. Scand J Med Sci Sports. 2009. PMID: 19706001 Review.
Cited by
-
In vivo monitoring of active subretinal fibrosis in mice using collagen hybridizing peptides.Lab Anim (NY). 2024 Aug;53(8):196-204. doi: 10.1038/s41684-024-01408-0. Epub 2024 Jul 26. Lab Anim (NY). 2024. PMID: 39060633 Free PMC article.
-
Blue-LIRIC in the rabbit cornea: efficacy, tissue effects, and repetition rate scaling.Biomed Opt Express. 2022 Mar 22;13(4):2346-2363. doi: 10.1364/BOE.448286. eCollection 2022 Apr 1. Biomed Opt Express. 2022. PMID: 35519279 Free PMC article.
-
Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program.Circ Res. 2019 Apr 12;124(8):1214-1227. doi: 10.1161/CIRCRESAHA.118.314438. Circ Res. 2019. PMID: 30686120 Free PMC article.
-
Osteochondral resurfacing implantation angle is more important than implant material stiffness.J Orthop Res. 2018 Nov;36(11):2911-2922. doi: 10.1002/jor.24101. Epub 2018 Jul 13. J Orthop Res. 2018. PMID: 29943463 Free PMC article.
-
Molecular and structural imaging in surgically induced murine osteoarthritis.Osteoarthritis Cartilage. 2020 Jul;28(7):874-884. doi: 10.1016/j.joca.2020.03.016. Epub 2020 Apr 16. Osteoarthritis Cartilage. 2020. PMID: 32305526 Free PMC article. Review.
References
-
- Tytherleigh-Strong G., Hirahara A. & Miniaci A. Rotator cuff disease. Curr. Opin. Rheumatol. 13, 135–145 (2001). - PubMed
-
- Nho S. J., Yadav H., Shindle M. K. & Macgillivray J. D. Rotator cuff degeneration: etiology and pathogenesis. Am. J. Sports Med. 36, 987–993 (2008). - PubMed
-
- Kraushaar B. S. & Nirschl R. P. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J. Bone Joint Surg. Am. 81, 259–278 (1999). - PubMed
-
- Leadbetter W. B. Cell-matrix response in tendon injury. Clin. Sports Med. 11, 533–578 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

