Ultrastructural alterations in Schistosoma mansoni juvenile and adult male worms after in vitro incubation with primaquine
- PMID: 28327785
- PMCID: PMC5354608
- DOI: 10.1590/0074-02760160324
Ultrastructural alterations in Schistosoma mansoni juvenile and adult male worms after in vitro incubation with primaquine
Abstract
Background: Praziquantel has been cited as the only drug for treating schistosomiasis. However, concerns over drug resistance have encouraged the search for novel drug leads. The antimalarial drug primaquine possesses interesting anti-schistosmal properties.
Objectives: This study is the first to document the potential role of primaquine as a schistosomicide and the ultrastructural changes induced by primaquine on juvenile or adult male worms of Schistosoma mansoni.
Methods: Ultrastructural alterations in the tegumental surface of 21-day-old juvenile and adult male worms of S. mansoni were demonstrated following primaquine treatment at different concentrations (2, 5, 10, 15, and 20 µg/mL) and incubation periods (1, 3, 6, 24, and 48 h) in vitro, using both scanning and transmission electron microscopy.
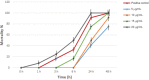
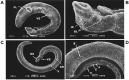
Findings: At low concentrations (2, 5, and 10 µg/mL) both juvenile and adult male worms were alive after 24 h of incubation, whereas contraction, paralysis, and death of all worms were observed after 24 h of drug exposure at 20 µg/mL. The tegument of juvenile and adult male worms treated with primaquine exhibited erosion, peeling, and sloughing. Furthermore, extensive damage of both tegumental and subtegumental layers included embedded spines, and shrinkage of muscles with vacuoles. The in vitro results confirmed that primaquine has dose-dependent effects with 20 µg/mL as the most effective concentration in a short incubation period.
Main conclusions: The schistosomicidal activity of primaquine indicates that this drug possesses moderate in vitro activity against juvenile and adult male worms, since it caused high mortality and tegumental alterations. This study confirmed that the antimalarial drug primaquine possesses anti-schistosomal activity. Further investigation is needed to elucidate its mechanism of action.
Figures





Similar articles
-
Anthelmintic activity in vitro and in vivo of Baccharis trimera (Less) DC against immature and adult worms of Schistosoma mansoni.Exp Parasitol. 2014 Apr;139:63-72. doi: 10.1016/j.exppara.2014.02.010. Epub 2014 Mar 3. Exp Parasitol. 2014. PMID: 24602876
-
Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni.Parasitology. 2010 Jan;137(1):85-98. doi: 10.1017/S0031182009990965. Epub 2009 Oct 9. Parasitology. 2010. PMID: 19814844
-
In vitro and in vivo anthelmintic activity of (-)-6,6'-dinitrohinokinin against schistosomula and juvenile and adult worms of Schistosoma mansoni.Acta Trop. 2015 Sep;149:195-201. doi: 10.1016/j.actatropica.2015.06.005. Epub 2015 Jun 10. Acta Trop. 2015. PMID: 26071648
-
Schistosoma mansoni: the effects of a subcurative dose of praziquantel on the ultrastructure of worms in vivo.Z Parasitenkd. 1983;69(1):73-90. doi: 10.1007/BF00934012. Z Parasitenkd. 1983. PMID: 6340360 Review.
-
Mefloquine, a new type of compound against schistosomes and other helminthes in experimental studies.Parasitol Res. 2013 Nov;112(11):3723-40. doi: 10.1007/s00436-013-3559-0. Epub 2013 Aug 27. Parasitol Res. 2013. PMID: 23979493 Review.
Cited by
-
Evaluation of metformin's effect on the adult and juvenile stages of Schistosoma mansoni: an in-vitro study.J Parasit Dis. 2025 Mar;49(1):69-83. doi: 10.1007/s12639-024-01731-w. Epub 2024 Sep 17. J Parasit Dis. 2025. PMID: 39975621
-
Botanical Products in the Treatment and Control of Schistosomiasis: Recent Studies and Distribution of Active Plant Resources According to Affected Regions.Biology (Basel). 2020 Aug 13;9(8):223. doi: 10.3390/biology9080223. Biology (Basel). 2020. PMID: 32823660 Free PMC article. Review.
-
Assessment of the potential therapeutic effects of omeprazole in Schistosoma mansoni infected mice.Parasitol Res. 2019 Dec;118(12):3399-3408. doi: 10.1007/s00436-019-06465-w. Epub 2019 Oct 26. Parasitol Res. 2019. PMID: 31655904
-
Microwave-assisted hydrothermal fabrication of hierarchical-stacked mesoporous decavanadate-intercalated ZnAl nanolayered double hydroxide to exterminate different developmental stages of Trichinella spiralis and Schistosoma mansoniin-vitro.Heliyon. 2023 Jul 8;9(7):e18110. doi: 10.1016/j.heliyon.2023.e18110. eCollection 2023 Jul. Heliyon. 2023. PMID: 37483817 Free PMC article.
-
Alendronate repositioning as potential anti-parasitic agent targeting Trichinella spiralis inorganic pyrophosphatase, in vitro supported molecular docking and molecular dynamics simulation study.BMC Chem. 2025 May 6;19(1):119. doi: 10.1186/s13065-025-01468-4. BMC Chem. 2025. PMID: 40329381 Free PMC article.
References
-
- Carneiro-Santos P, Thornhill JA, Doenhoff MJ, Hagan P, Kusel JR. Acidic vesicles of Schistosoma mansoni. Parasitol Res. 2001;87(12):1001–1006. - PubMed
-
- Cheng L, Lei L, Guo S, Zhu C, Rong H, Guo D, et al. Schistosoma japonicum. Treatment of different developmental stages in mice with long-acting praziquantel implants. Exp Parasitol. 2011;129(3):254–259. - PubMed
-
- Clegg JA, Smithers SR. The effects of immune rhesus monkey serum on schistosomula of Schistosoma mansoni during cultivation in vitro. Int J Parasitol. 1972;2(1):79–98. - PubMed
-
- Oliveira RN de, Rehder VLG, Oliveira ASS, Montanari JI, Jeraldo VL, Linhares AX, et al. Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (Less) DC. Exp Parasitol. 2012;132(2):135–143. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

