Final overall survival analysis for the phase II RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC
- PMID: 28327953
- PMCID: PMC5452072
- DOI: 10.1093/annonc/mdx075
Final overall survival analysis for the phase II RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC
Erratum in
-
Final overall survival analysis for the phase II RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC.Ann Oncol. 2018 Nov 1;29(11):2269. doi: 10.1093/annonc/mdx807. Ann Oncol. 2018. PMID: 29390043 Free PMC article. No abstract available.
Abstract
Background: RECORD-3 compared everolimus and sunitinib as first-line therapy, and the sequence of everolimus followed by sunitinib at progression compared with the opposite (standard) sequence in patients with metastatic renal cell carcinoma (mRCC). This final overall survival (OS) analysis evaluated mature data for secondary end points.
Patients and methods: Patients received either first-line everolimus followed by second-line sunitinib at progression (n = 238) or first-line sunitinib followed by second-line everolimus (n = 233). Secondary end points were combined first- and second-line progression-free survival (PFS), OS, and safety. The impacts of neutrophil lymphocyte ratio (NLR) and baseline levels of soluble biomarkers on OS were explored.
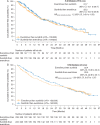
Results: At final analysis, median duration of exposure was 5.6 months for everolimus and 8.3 months for sunitinib. Median combined PFS was 21.7 months [95% confidence interval (CI) 15.1-26.7] with everolimus-sunitinib and 22.2 months (95% CI 16.0-29.8) with sunitinib-everolimus [hazard ratio (HR)EVE-SUN/SUN-EVE, 1.2; 95% CI 0.9-1.6]. Median OS was 22.4 months (95% CI 18.6-33.3) for everolimus-sunitinib and 29.5 months (95% CI 22.8-33.1) for sunitinib-everolimus (HREVE-SUN/SUN-EVE, 1.1; 95% CI 0.9-1.4). The rates of grade 3 and 4 adverse events suspected to be related to second-line therapy were 47% with everolimus and 57% with sunitinib. Higher NLR and 12 soluble biomarker levels were identified as prognostic markers for poor OS with the association being largely independent of treatment sequences.
Conclusions: Results of this final OS analysis support the sequence of sunitinib followed by everolimus at progression in patients with mRCC. The safety profiles of everolimus and sunitinib were consistent with those previously reported, and there were no unexpected safety signals.
Clinical trials number: ClinicalTrials.gov identifier, NCT00903175.
Keywords: everolimus; renal cell carcinoma; sequential targeted therapy; sunitinib.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures

References
-
- Escudier B, Porta C, Schmidinger M. et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25 (Suppl 3): iii49–iii56. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer—v1.2017. https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. (2 March 2017, date last accessed)
-
- Neuenschwander B, Rouyrre N, Hollaender N. et al. A proof of concept phase II non-inferiority criterion. Stat Med 2011; 30: 1618–1627. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

