Carbon dating cancer: defining the chronology of metastatic progression in colorectal cancer
- PMID: 28327965
- PMCID: PMC5452067
- DOI: 10.1093/annonc/mdx074
Carbon dating cancer: defining the chronology of metastatic progression in colorectal cancer
Abstract
Background: Patients often ask oncologists how long a cancer has been present before causing symptoms or spreading to other organs. The evolutionary trajectory of cancers can be defined using phylogenetic approaches but lack of chronological references makes dating the exact onset of tumours very challenging.
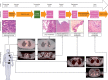
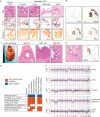
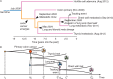
Patients and methods: Here, we describe the case of a colorectal cancer (CRC) patient presenting with synchronous lung metastasis and metachronous thyroid, chest wall and urinary tract metastases over the course of 5 years. The chest wall metastasis was caused by needle tract seeding, implying a known time of onset. Using whole genome sequencing data from primary and metastatic sites we inferred the complete chronology of the cancer by exploiting the time of needle tract seeding as an in vivo 'stopwatch'. This approach allowed us to follow the progression of the disease back in time, dating each ancestral node of the phylogenetic tree in the past history of the tumour. We used a Bayesian phylogenomic approach, which accounts for possible dynamic changes in mutational rate, to reconstruct the phylogenetic tree and effectively 'carbon date' the malignant progression.
Results: The primary colon cancer emerged between 5 and 8 years before the clinical diagnosis. The primary tumour metastasized to the lung and the thyroid within a year from its onset. The thyroid lesion presented as a tumour-to-tumour deposit within a benign Hurthle adenoma. Despite rapid metastatic progression from the primary tumour, the patient showed an indolent disease course. Primary cancer and metastases were microsatellite stable and displayed low chromosomal instability. Neo-antigen analysis suggested minimal immunogenicity.
Conclusion: Our data provide the first in vivo experimental evidence documenting the timing of metastatic progression in CRC and suggest that genomic instability might be more important than the metastatic potential of the primary cancer in dictating CRC fate.
Keywords: cancer evolution; metastatic colorectal carcinoma; mutational analysis; phylogenetic tree; synchronous metastases; whole genome sequencing.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Comment in
-
Tracking colorectal cancer evolution in time and space.Ann Oncol. 2017 Jun 1;28(6):1163-1165. doi: 10.1093/annonc/mdx127. Ann Oncol. 2017. PMID: 28383707 No abstract available.
References
-
- Van Cutsem E, Cervantes A, Nordlinger B. et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl 3): iii1–iii9. - PubMed
-
- Ghiringhelli F, Hennequin A, Drouillard A. et al. Epidemiology and prognosis of synchronous and metachronous colon cancer metastases: a French population-based study. Dig Liver Dis 2014; 46: 854–858. - PubMed
-
- Tyagi R, Dey P.. Needle tract seeding: an avoidable complication. Diagn Cytopathol 2014; 42: 636–640. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

