Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment of anemia of chronic inflammation
- PMID: 28328181
- PMCID: PMC7732206
- DOI: 10.1111/hdi.12543
Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment of anemia of chronic inflammation
Abstract
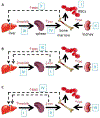
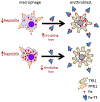
Anemia of chronic inflammation (ACI) is a frequently diagnosed anemia and portends an independently increased morbidity and poor outcome associated with multiple underlying diseases. The pathophysiology of ACI is multifactorial, resulting from the effects of inflammatory cytokines which both directly and indirectly suppress erythropoiesis. Recent advances in molecular understanding of iron metabolism provide strong evidence that immune mediators, such as IL-6, lead to hepcidin-induced hypoferremia, iron sequestration, and decreased iron availability for erythropoiesis. The role of hepcidin-ferroportin axis in the pathophysiology of ACI is stimulating the development of new diagnostics and targeted therapies. In this review, we present an overview of and rationale for inflammation-, iron-, and erythropoiesis-related strategies currently in development.
Keywords: Anemia; inflammation; iron metabolism.
© 2017 International Society for Hemodialysis.
Conflict of interest statement
Figures
Similar articles
-
Iron sequestration and anemia of inflammation.Semin Hematol. 2009 Oct;46(4):387-93. doi: 10.1053/j.seminhematol.2009.06.001. Semin Hematol. 2009. PMID: 19786207 Free PMC article. Review.
-
Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation.Am J Hematol. 2012 Apr;87(4):392-400. doi: 10.1002/ajh.23110. Epub 2012 Jan 31. Am J Hematol. 2012. PMID: 22290531 Free PMC article. Review.
-
Anemia of chronic disease: a unique defect of iron recycling for many different chronic diseases.Eur J Intern Med. 2014 Jan;25(1):12-7. doi: 10.1016/j.ejim.2013.07.011. Epub 2013 Aug 26. Eur J Intern Med. 2014. PMID: 23988263 Review.
-
Hepcidin-ferroportin axis in health and disease.Vitam Horm. 2019;110:17-45. doi: 10.1016/bs.vh.2019.01.002. Epub 2019 Feb 8. Vitam Horm. 2019. PMID: 30798811 Free PMC article. Review.
-
Anemia of Inflammation: A Review.Med Clin North Am. 2017 Mar;101(2):285-296. doi: 10.1016/j.mcna.2016.09.005. Epub 2016 Dec 24. Med Clin North Am. 2017. PMID: 28189171 Free PMC article. Review.
Cited by
-
Increased Outdoor PM2.5 Concentration Is Associated with Moderate/Severe Anemia in Children Aged 6-59 Months in Lima, Peru.J Environ Public Health. 2019 Jul 24;2019:6127845. doi: 10.1155/2019/6127845. eCollection 2019. J Environ Public Health. 2019. PMID: 31428166 Free PMC article.
-
Multiple Infections, Nutrient Deficiencies, and Inflammation as Determinants of Anemia and Iron Status during Pregnancy: The MINDI Cohort.Nutrients. 2024 Jun 2;16(11):1748. doi: 10.3390/nu16111748. Nutrients. 2024. PMID: 38892681 Free PMC article.
-
Empyema thoracis complicated by anaemia as caused by occult bonelet aspiration.Respirol Case Rep. 2020 Sep 10;8(8):e00661. doi: 10.1002/rcr2.661. eCollection 2020 Nov. Respirol Case Rep. 2020. PMID: 32995011 Free PMC article.
-
Iron deficiency as therapeutic target in heart failure: a translational approach.Heart Fail Rev. 2020 Mar;25(2):173-182. doi: 10.1007/s10741-019-09815-z. Heart Fail Rev. 2020. PMID: 31230175 Review.
-
TGFβ2-Hepcidin Feed-Forward Loop in the Trabecular Meshwork Implicates Iron in Glaucomatous Pathology.Invest Ophthalmol Vis Sci. 2020 Mar 9;61(3):24. doi: 10.1167/iovs.61.3.24. Invest Ophthalmol Vis Sci. 2020. PMID: 32182331 Free PMC article.
References
-
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005; 352:1011–1023. - PubMed
-
- Matzner Y, Levy S, Grossowicz N, Izak G, Hershko C. Prevalence and causes of anemia in elderly hospitalized patients. Gerontology. 1979; 25:113–119. - PubMed
-
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010; 116: 1574–1584. - PubMed
-
- Cazzola M, Ponchio L, de Benedetti F, et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996; 87:4824–4830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

