Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study
- PMID: 28328302
- PMCID: PMC5946724
- DOI: 10.1200/JCO.2016.70.1524
Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study
Abstract
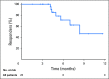
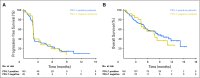
Purpose There are no approved treatments for recurrent/metastatic head and neck squamous cell carcinoma refractory to platinum and cetuximab. In the single-arm, phase II KEYNOTE-055 study, we evaluated pembrolizumab, an anti-programmed death 1 receptor antibody, in this platinum- and cetuximab-pretreated population with poor prognosis. Methods Eligibility stipulated disease progression within 6 months of platinum and cetuximab treatment. Patients received pembrolizumab 200 mg every 3 weeks. Imaging was performed every 6 to 9 weeks. Primary end points: overall response rate (Response Evaluation Criteria in Solid Tumors v1.1, central review) and safety. Efficacy was assessed in all dosed patients and in subgroups on the basis of programmed death ligand 1 (PD-L1) expression and human papillomavirus (HPV) status. Results Among 171 patients treated, 75% received two or more prior lines of therapy for metastatic disease, 82% were PD-L1 positive, and 22% were HPV positive. At the time of analysis, 109 patients (64%) experienced a treatment-related adverse event; 26 patients (15%) experienced a grade ≥ 3 event. Seven patients (4%) discontinued treatment, and one died of treatment-related adverse events. Overall response rate was 16% (95% CI, 11% to 23%), with a median duration of response of 8 months (range, 2+ to 12+ months); 75% of responses were ongoing at the time of analysis. Response rates were similar in all HPV and PD-L1 subgroups. Median progression-free survival was 2.1 months, and median overall survival was 8 months. Conclusion Pembrolizumab exhibited clinically meaningful antitumor activity and an acceptable safety profile in recurrent/metastatic head and neck squamous cell carcinoma previously treated with platinum and cetuximab.
Figures



References
-
- Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4:156–171. - PubMed
-
- Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13:35–46. - PubMed
-
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. - PubMed
-
- Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

