Overall survival of patients with KRAS wild-type tumor treated with FOLFOX/FORFIRI±cetuximab as the first-line treatment for metastatic colorectal cancer: A meta-analysis
- PMID: 28328812
- PMCID: PMC5371449
- DOI: 10.1097/MD.0000000000006335
Overall survival of patients with KRAS wild-type tumor treated with FOLFOX/FORFIRI±cetuximab as the first-line treatment for metastatic colorectal cancer: A meta-analysis
Abstract
The addition of cetuximab to FOLFIRI or FOLFOX as the first-line treatment for metastatic colorectal cancer (mCRC) was shown to reduce the risk of disease progression and increase the chance of response in patients with KRAS wild-type disease. An updated systematic meta-analysis was undertaken to determine the efficacy of cetuximab plus FOLFIRI or FOLFOX.Major databases were searched to identify RCTs investigating wild-type KRAS mCRC after the first-line treatment, and treatment with FOLFOX/FORFIRI ± cetuximab was compared. Data on clinical efficacy and safety were pooled and compared by ORs, HRs, and 95% CIs.Five eligible trials with 1464 patients were included in the meta-analysis. Compared to FOLFOX/FORFIRI, cetuximab as the first-line therapy has improved overall survival (OS) (hazard ratio [HR] = 0.82, 95% confidence interval [CI]: 0.72-0.93, P = 0.003), progression-free survival (PFS) (HR = 0.66, 95% CI: 0.56 -0.77, P < 0.00001), and overall response rate (ORR) (odds ratio [OR] = 2.12, 95% CI: 1.70-2.65, P < 0.00001). However, Grade 3/4 AE was increased with the OR of 2.76 (95%CI: 2.01-3.78, P < 0.00001). The most common grade 3/4 toxicity in the wild-type KRAS population was neutropenia and diarrhea. For cetuximab plus FOLFIRI, there was a higher incidence of grade 3 or 4 diarrhea (OR = 1.76, 95% CI: 1.15-2.70, P = 0.01), but there was no significant difference for neutropenia (OR = 1.35, 95% CI: 1.00-1.83, P = 0.05).The addition of cetuximab in mCRC as the first-line treatment is a potential effective approach in the improved outcomes but associated with increased toxicity.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
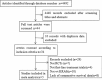
Figures





References
-
- Lyseng-Williamson KA. Cetuximab: a guide to its use in combination with FOLFIRI in the first-line treatment of metastatic colorectal cancer in the USA. Mol Diagn Ther 2012;16:317–22. - PubMed
-
- Ooki A, Ando M, Sakamoto J, et al. A prospective observational study to examine the relationship between quality of life and adverse events of first-line chemotherapy plus cetuximab in patients with KRAS wild-type unresectable metastatic colorectal cancer: QUACK Trial. Jpn J Clin Oncol 2014;44:383–7. - PubMed
-
- Bokemeyer C, Bondarenko I, Hartmann JT, et al. Overall survival of patients with KRAS wild-type tumours treated with FOLFOX4 +/− cetuximab as 1st-line treatment for metastatic colorectal cancer: the OPUS study. EJC Suppl 2009;7:346–1346.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

