Therapeutic Vaccine for Genital Herpes Simplex Virus-2 Infection: Findings From a Randomized Trial
- PMID: 28329211
- PMCID: PMC7206854
- DOI: 10.1093/infdis/jix004
Therapeutic Vaccine for Genital Herpes Simplex Virus-2 Infection: Findings From a Randomized Trial
Abstract
Background: Genital herpes simplex virus type 2 (HSV-2) infection causes recurrent lesions and frequent viral shedding. GEN-003 is a candidate therapeutic vaccine containing HSV-2 gD2∆TMR and ICP4.2, and Matrix-M2 adjuvant.
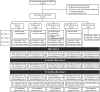
Methods: Persons with genital herpes were randomized into 3 dose cohorts to receive 3 intramuscular doses 21 days apart of 10 µg, 30 µg, or 100 µg of GEN-003, antigens without adjuvant, or placebo. Participants obtained genital swab specimens twice daily for HSV-2 detection and monitored genital lesions for 28-day periods at baseline and at intervals after the last dose.
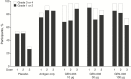
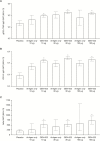
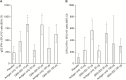
Results: One hundred and thirty-four persons received all 3 doses. Reactogenicity was associated with adjuvant but not with antigen dose or dose number. No serious adverse events were attributed to GEN-003. Compared with baseline, genital HSV-2 shedding rates immediately after dosing were reduced with GEN-003 (from 13.4% to 6.4% for 30 μg [P < .001] and from 15.0% to 10.3% for 100 µg [P < .001]). Lesion rates were also significantly (P < .01) reduced immediately following immunization with 30 µg or 100 µg of GEN-003. GEN-003 elicited increases in antigen binding, virus neutralizing antibody, and T-cell responses.
Conclusions: GEN-003 had an acceptable safety profile and stimulated humoral and cellular immune responses. GEN-003 at doses of 30 µg and 100 µg reduced genital HSV shedding and lesion rates.
Clinical trials registration: NCT01667341 (funded by Genocea).
Keywords: GEN-003; Genital herpes; herpes simplex virus type 2 infection; therapeutic vaccine; immunotherapy.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
Comment in
-
Vaccination to Reduce Reactivation of Herpes Simplex Virus Type 2.J Infect Dis. 2017 Mar 15;215(6):844-846. doi: 10.1093/infdis/jix006. J Infect Dis. 2017. PMID: 28453833 Free PMC article. No abstract available.
References
-
- Corey L, Wald A, Patel R, et al. ; Valacyclovir HSV Transmission Study Group. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350:11–20. - PubMed
-
- Long D, Skoberne M, Gierahn TM, et al. Identification of novel virus-specific antigens by CD4⁺ and CD8⁺ T cells from asymptomatic HSV-2 seropositive and seronegative donors. Virology 2014; 464–465:296–311. - PubMed
-
- Cox RJ, Pedersen G, Madhun AS, et al. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M™ adjuvant in a phase I clinical trial. Vaccine 2011; 29:8049–59. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

