Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life
- PMID: 28329707
- PMCID: PMC5415308
- DOI: 10.1016/j.immuni.2017.02.019
Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life
Abstract
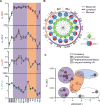
Maturation and migration to lymph nodes (LNs) constitutes a central paradigm in conventional dendritic cell (cDC) biology but remains poorly defined in humans. Using our organ donor tissue resource, we analyzed cDC subset distribution, maturation, and migration in mucosal tissues (lungs, intestines), associated lymph nodes (LNs), and other lymphoid sites from 78 individuals ranging from less than 1 year to 93 years of age. The distribution of cDC1 (CD141hiCD13hi) and cDC2 (Sirp-α+CD1c+) subsets was a function of tissue site and was conserved between donors. We identified cDC2 as the major mature (HLA-DRhi) subset in LNs with the highest frequency in lung-draining LNs. Mature cDC2 in mucosal-draining LNs expressed tissue-specific markers derived from the paired mucosal site, reflecting their tissue-migratory origin. These distribution and maturation patterns were largely maintained throughout life, with site-specific variations. Our findings provide evidence for localized DC tissue surveillance and reveal a lifelong division of labor between DC subsets, with cDC2 functioning as guardians of the mucosa.
Keywords: dendritic cells; human immunology; mucosal immunity; tissue immunity.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Of Human DC Migrants and Residents.Immunity. 2017 Mar 21;46(3):342-344. doi: 10.1016/j.immuni.2017.03.006. Immunity. 2017. PMID: 28329699
References
-
- Aliberti J, Schulz O, Pennington DJ, Tsujimura H, Sousa CRE, Ozato K, Sher A. Essential role for ICSBP in the in vivo development of murine CD8 alpha(+) dendritic cells. Blood. 2003;101:305–310. - PubMed
-
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

