LACTB is a tumour suppressor that modulates lipid metabolism and cell state
- PMID: 28329758
- PMCID: PMC6246920
- DOI: 10.1038/nature21408
LACTB is a tumour suppressor that modulates lipid metabolism and cell state
Abstract
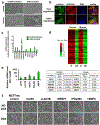
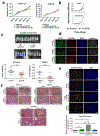
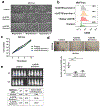
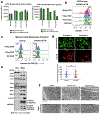
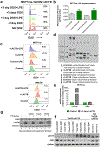
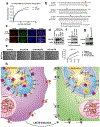
Post-mitotic, differentiated cells exhibit a variety of characteristics that contrast with those of actively growing neoplastic cells, such as the expression of cell-cycle inhibitors and differentiation factors. We hypothesized that the gene expression profiles of these differentiated cells could reveal the identities of genes that may function as tumour suppressors. Here we show, using in vitro and in vivo studies in mice and humans, that the mitochondrial protein LACTB potently inhibits the proliferation of breast cancer cells. Its mechanism of action involves alteration of mitochondrial lipid metabolism and differentiation of breast cancer cells. This is achieved, at least in part, through reduction of the levels of mitochondrial phosphatidylserine decarboxylase, which is involved in the synthesis of mitochondrial phosphatidylethanolamine. These observations uncover a novel mitochondrial tumour suppressor and demonstrate a connection between mitochondrial lipid metabolism and the differentiation program of breast cancer cells, thereby revealing a previously undescribed mechanism of tumour suppression.
Figures









Comment in
-
Tumour Suppressors: Following the clues of cancer-resistant tissues.Nat Rev Cancer. 2017 Apr 24;17(5):269. doi: 10.1038/nrc.2017.29. Nat Rev Cancer. 2017. PMID: 28436471 No abstract available.
-
LACTB-mediated tumour suppression by increased mitochondrial lipid metabolism.Cell Death Differ. 2017 Jul;24(7):1137-1139. doi: 10.1038/cdd.2017.60. Epub 2017 May 5. Cell Death Differ. 2017. PMID: 28475178 Free PMC article. No abstract available.
-
Commentary: LACTB is a tumour suppressor that modulates lipid metabolism and cell state.Front Physiol. 2017 Jun 8;8:396. doi: 10.3389/fphys.2017.00396. eCollection 2017. Front Physiol. 2017. PMID: 28642719 Free PMC article. No abstract available.
References
-
- Seely S Possible reasons for the high resistance of muscle to cancer. Med. Hypotheses 6, 133–137 (1980). - PubMed
-
- Walsh K & Perlman H Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev 7, 597–602 (1997). - PubMed
-
- Lassar AB, Skapek SX & Novitch B Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr. Opin. Cell Biol 6, 788–794 (1994). - PubMed
-
- Kasid A, Lippman ME, Papageorge AG, Lowy DR & Gelmann E P Transfection of v-rasH DNA into MCF-7 human breast cancer cells bypasses dependence on estrogen for tumorigenicity. Science 228, 725–728 (1985). - PubMed
-
- Smith TS et al. Identification, genomic organization, and mRNA expression of LACTB, encoding a serine β-lactamase-like protein with an amino-terminal transmembrane domain. Genomics 78, 12–14 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

