Doxorubicin Has Dose-Dependent Toxicity on Mouse Ovarian Follicle Development, Hormone Secretion, and Oocyte Maturation
- PMID: 28329872
- PMCID: PMC6074798
- DOI: 10.1093/toxsci/kfx047
Doxorubicin Has Dose-Dependent Toxicity on Mouse Ovarian Follicle Development, Hormone Secretion, and Oocyte Maturation
Abstract
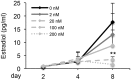
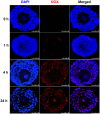
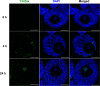
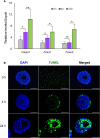
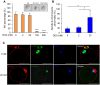
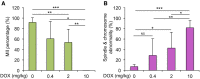
Doxorubicin (DOX), one of the most commonly used anticancer medications, has been reported to affect fertility by damaging ovarian follicles; however, the dose-dependent toxicity of DOX on the dynamic follicle development and oocyte maturation has not been well-defined. Our objective is to determine the effects of human-relevant exposure levels of DOX on follicular functions across developmental time. In vitro cultured multilayered secondary mouse follicles were treated with DOX at 0, 2, 20, 100, and 200 nM for 24 h, and follicle development, hormone secretion, and oocyte maturation were analyzed. DOX caused dose-dependent toxicity on follicle growth, survival, and secretion of 17β-estradiol (E2). At 200 nM, DOX induced DNA damage and apoptosis in follicle somatic cells first and then in oocytes, which was correlated with the uptake of DOX first to the somatic cells followed by germ cells. Follicles treated with DOX at 0, 2, and 20 nM showed similar oocyte metaphase II (MII) percentages after in vitro oocyte maturation; however, 20 nM DOX significantly increased the number of MII oocytes with abnormal spindle morphology and chromosome misalignment. In an effort to harmonize the in vitro study to in vivo treatment, dose-dependent toxicity on oocyte meiotic maturation was found in 16-day-old CD-1 mice treated with DOX at 0, 0.4, 2, and 10 mg/kg, consistent with the in vitro oocyte maturation outcomes. Our study demonstrates that DOX has dose-dependent toxicity on ovarian follicle development, hormone secretion, and oocyte maturation, which are three key factors to support the female reproductive and endocrine functions.
Keywords: doxorubicin; follicle development; oocyte maturation; ovarian toxicity.
© The Author 2017. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Ahn R. W., Barrett S. L., Raja M. R., Jozefik J. K., Spaho L., Chen H. M., Bally M. B., Mazar A. P., Avram M. J., Winter J. N., et al. (2013). Nano-encapsulation of arsenic trioxide enhances efficacy against murine lymphoma model while minimizing its impact on ovarian reserve in vitro and in vivo. PloS One 8. - PMC - PubMed
-
- Bar-Joseph H., Ben-Aharon I., Rizel S., Stemmer S. M., Tzabari M., Shalgi R. (2010). Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod. Toxicol. 30, 566–572. - PubMed
-
- Barpe D. R., Rosa D. D., Froehlich P. E. (2010). Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur. J. Pharm. Sci. 41, 458–463. - PubMed
-
- Bines J., Oleske D. M., Cobleigh M. A. (1996). Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 14, 1718–1729. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

