Purification and characterization of the enzymes involved in nicotinamide adenine dinucleotide degradation by Penicillium brevicompactum NRC 829
- PMID: 28330104
- PMCID: PMC4722047
- DOI: 10.1007/s13205-015-0349-7
Purification and characterization of the enzymes involved in nicotinamide adenine dinucleotide degradation by Penicillium brevicompactum NRC 829
Abstract
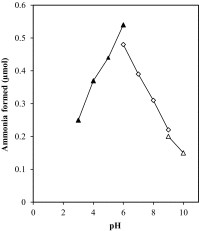
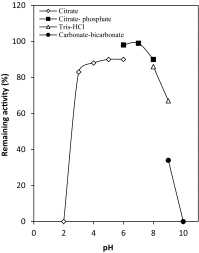
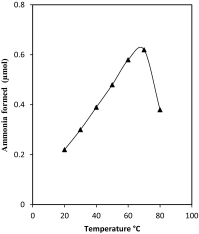
The present study was conducted to investigate a new pathway for the degradation of nicotinamide adenine dinucleotide (NAD) by Penicillium brevicompactum NRC 829 extracts. Enzymes involved in the hydrolysis of NAD, i.e. alkaline phosphatase, aminohydrolase and glycohydrolase were determined. Alkaline phosphatase was found to catalyse the sequential hydrolysis of two phosphate moieties of NAD molecule to nicotinamide riboside plus adenosine. Adenosine was then deaminated by aminohydrolase to inosine and ammonia. While glycohydrolase catalyzed the hydrolysis of the nicotinamide-ribosidic bond of NAD+ to produce nicotinamide and ADP-ribose in equimolar amounts, enzyme purification through a 3-step purification procedure revealed the existence of two peaks of alkaline phosphatases, and one peak contained deaminase and glycohydrolase activities. NAD deaminase was purified to homogeneity as estimated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis with an apparent molecular mass of 91 kDa. Characterization and determination of some of NAD aminohydrolase kinetic properties were conducted due to its biological role in the regulation of cellular NAD level. The results also revealed that NAD did not exert its feedback control on nicotinamide amidase produced by P. brevicompactum.
Keywords: Alkaline phosphatases; Deaminase; Glycohydrolase; NAD degradation; Penicillium brevicompactum NRC 829.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures



Similar articles
-
Nicotinamide 2-fluoroadenine dinucleotide unmasks the NAD+ glycohydrolase activity of Aplysia californica adenosine 5'-diphosphate ribosyl cyclase.Biochemistry. 2007 Apr 3;46(13):4100-9. doi: 10.1021/bi061933w. Epub 2007 Mar 7. Biochemistry. 2007. PMID: 17341094
-
Modulation by nicotinamide adenine dinucleotide of sympathetic and sensory-motor neurotransmission via P1-purinoceptors in the rat mesenteric arterial bed.Br J Pharmacol. 1995 Apr;114(8):1541-8. doi: 10.1111/j.1476-5381.1995.tb14937.x. Br J Pharmacol. 1995. PMID: 7599921 Free PMC article.
-
Purification and properties of a mitochondrial NAD+ glycohydrolase.Arch Biochem Biophys. 1983 Jul 1;224(1):358-64. doi: 10.1016/0003-9861(83)90220-5. Arch Biochem Biophys. 1983. PMID: 6870260
-
Identification of bovine liver mitochondrial NAD+ glycohydrolase as ADP-ribosyl cyclase.Biochem J. 1997 Sep 1;326 ( Pt 2)(Pt 2):401-5. doi: 10.1042/bj3260401. Biochem J. 1997. PMID: 9291111 Free PMC article.
-
[Role of adenine mono- and dinucleotides in ammonia formation in brain tissue].Vopr Biokhim Mozga. 1975;10:5-32. Vopr Biokhim Mozga. 1975. PMID: 186942 Review. Russian.
Cited by
-
The essential schistosome tegumental ectoenzyme SmNPP5 can block NAD-induced T cell apoptosis.Virulence. 2020 Dec;11(1):568-579. doi: 10.1080/21505594.2020.1770481. Virulence. 2020. PMID: 32441549 Free PMC article.
References
-
- Ali TH, Haroun BM, Tantawy AE. Optimization of culture conditions for the production of NAD-degrading enzymes by Aspergillus oryzae NRRL 447. Adv Food Sci. 2012;34(4):195–201.
-
- Ashok KJ, Pinto GJ, Kavitha AK, Palathra MJ. The diagnostic and prognostic value of serum adenosine deaminase levels in head and neck cancer. J Clin Diagn Res. 2008;3:833–837.
-
- Bi M, Koklu S, Meric Y, Basar O, Yilmaz G, Yuksel O, Yildirim E, Ozturk Z. Serum adenosine deaminase levels in pancreatic diseases. Pancreat. 2007;7:5–6. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

