Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacillus caseinilyticus
- PMID: 28330122
- PMCID: PMC4752951
- DOI: 10.1007/s13205-016-0377-y
Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacillus caseinilyticus
Abstract
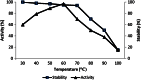
Alkaline proteases are important enzymes in many industrial applications, especially as additives in laundry detergent industry. Though there are a number of Bacillus species which are reported to be producing proteases, the efficiency of a protease produced by a novel strain has to be studied in comparison to the others. Hence, in this study, an alkaline serine protease produced by a novel species Bacillus caseinilyticus was purified and characterized for its possible usage in detergent industry. Ammonium sulphate, dialysis and DEAE column chromatographic methods were used for purification of the isolated alkaline protease. The molecular weight of the protease was determined by SDS-PAGE and it was found to be 66 kDa. Peptide mass fingerprinting (PMF) was carried out using MALDI-TOF-TOF mass spectrometry and the peptides were found to be similar to that of subtilisin protease. Specific activity of purified protein was found to be 89.2 U/mg. Optimum pH and temperature for enzyme activity were at pH 8 and 60 °C, respectively, showing stability with 10 mM CaCl2. Phenyl methyl sulphonyl fluoride (PMSF) at both 5 and 10 mM concentrations completely inhibited the enzyme activity suggesting its serine nature. EDTA, metal ions Mg2+ and Ca2+ increased the enzyme activity. The one factor at a time optimisation of the protease production was carried to identify the important factors that affect its production. After optimisation, the protease was produced at lab scale, purified and characterised. This alkali, thermotolerant serine protease was found to be significantly stable in the presence of various surfactants and H2O2. Also, it was successfully able to remove blood stain when used as an additive along with commercial detergent suggesting its potential application in the laundry detergent industry.
Keywords: Application; Assay; Laundry detergent industry; Protease.
Conflict of interest statement
The authors declare that there is no conflict of interest on publication of this article.
Figures



References
-
- Agarwal D, Patidar P, Banerjee T, Pati S. Production of alkaline protease by Penicillium sp. under SSF conditions and its application to soy protein hydrolysis. Process Biochem. 2004;3:977–982. doi: 10.1016/S0032-9592(03)00212-7. - DOI
-
- Annamalai N, Rajeswari MV, Thavasi R, Vijayalakshmi S, Balasubramanian T. Optimization, purification and characterization of novel thermostable, haloalkaline, solvent stable protease from Bacillus halodurans CAS6 using marine shellfish wastes: a potential additive for detergent and antioxidant synthesis. Bioprocess Biosys Engg. 2013;36:873–883. doi: 10.1007/s00449-012-0820-3. - DOI - PubMed
-
- Banerjee UC, Sani RK, Azmi W, Soni R. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem. 1999;35:213–219. doi: 10.1016/S0032-9592(99)00053-9. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

