Purification and characterization of a surfactant-compatible lipase from Aspergillus tamarii JGIF06 exhibiting energy-efficient removal of oil stains from polycotton fabric
- PMID: 28330188
- PMCID: PMC4909032
- DOI: 10.1007/s13205-016-0449-z
Purification and characterization of a surfactant-compatible lipase from Aspergillus tamarii JGIF06 exhibiting energy-efficient removal of oil stains from polycotton fabric
Abstract
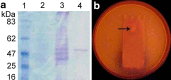
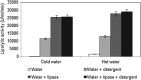
An extracellular lipase with 23,666.66 U/ml/min activity was produced by Aspergillus tamarii JGIF06 under submerged fermentation in mineral salt medium containing coconut oil (2.5 % v/v), tryptone (2 % w/v) and ammonium chloride (2 % w/v), with initial pH of 5 ± 0.2, incubated at 25 °C for 7 days on a rotary shaker at 120 rpm. A 7.9-fold increase in lipase-specific activity was recorded after purification by DEAE Sepharose ion exchange and Sephadex G200 column chromatography. The apparent molecular mass of this enzyme was revealed as 50 kDa by sodium dodecyl sulphate polyacrylamide gel electrophoresis. The optimal lipase activity was recorded at pH 4 and 37 °C. The enzyme revealed broad specificity towards different vegetable oils. The K m and V max of the lipase on olive oil was found to be 330.4 mg and 53,690 U/ml/min, respectively. The lipase activity was stable in the presence of surfactants such as cetrimonium bromide, sodium dodecyl sulphate and Tween 80, and metal ions and reagents such as Ca2+, Ba2+ and 2-mercaptoethanol. However, the activity was greatly reduced in the presence of organic solvents such as chloroform. The stain removal potential of the crude lipase was determined on polycotton fabric pieces stained with peanut oil. Lipase added to cold water alone significantly enhanced the removal of stain by 152 %. The addition of lipase also improved the stain removal efficiency of a commercially available detergent in the presence of either cold (25 ± 2 °C) or hot (65 ± 2 °C) water. The current findings suggest the potentiality of this enzyme for energy-efficient biocatalytic application.
Keywords: Aspergillus tamarii; Lipase; Surfactant; Vegetable oil.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Coconut oil induced production of a surfactant-compatible lipase from Aspergillus tamarii under submerged fermentation.J Basic Microbiol. 2017 Feb;57(2):114-120. doi: 10.1002/jobm.201600478. Epub 2016 Oct 6. J Basic Microbiol. 2017. PMID: 27709654
-
Optimization of process parameters influencing the submerged fermentation of extracellular lipases from Pseudomonas aeruginosa, candida albicans and Aspergillus flavus.Pak J Biol Sci. 2011 Nov 15;14(22):1011-8. doi: 10.3923/pjbs.2011.1011.1018. Pak J Biol Sci. 2011. PMID: 22514878
-
Screening, purification, and characterization of an extracellular lipase from Aureobasidium pullulans isolated from stuffed buns steamers.J Zhejiang Univ Sci B. 2019 Apr.;20(4):332-342. doi: 10.1631/jzus.B1800213. J Zhejiang Univ Sci B. 2019. PMID: 30932378 Free PMC article.
-
Isolation and characterization of a thermophilic lipase from bacillus thermoleovorans ID-1.FEMS Microbiol Lett. 1999 Oct 15;179(2):393-400. doi: 10.1111/j.1574-6968.1999.tb08754.x. FEMS Microbiol Lett. 1999. PMID: 10518742
-
Lipase from marine Aspergillus awamori BTMFW032: production, partial purification and application in oil effluent treatment.N Biotechnol. 2011 Oct;28(6):627-38. doi: 10.1016/j.nbt.2011.04.007. Epub 2011 Apr 27. N Biotechnol. 2011. PMID: 21549226
Cited by
-
Production of alkaline lipase by Aspergillus terreus AUMC 15762 for laundry application.AMB Express. 2025 Apr 13;15(1):64. doi: 10.1186/s13568-025-01865-x. AMB Express. 2025. PMID: 40223016 Free PMC article.
-
Statistical optimization of cultural medium composition of thermoalkalophilic lipase produced by a chemically induced mutant strain of Bacillus atrophaeus FSHM2.3 Biotech. 2019 Jul;9(7):268. doi: 10.1007/s13205-019-1789-2. Epub 2019 Jun 15. 3 Biotech. 2019. PMID: 31218179 Free PMC article.
-
Lipase from Rhizopus oryzae R1: in-depth characterization, immobilization, and evaluation in biodiesel production.J Genet Eng Biotechnol. 2021 Jan 5;19(1):1. doi: 10.1186/s43141-020-00094-y. J Genet Eng Biotechnol. 2021. PMID: 33400043 Free PMC article.
-
Characterization of Detergent-Compatible Lipases from Candida albicans and Acremonium sclerotigenum under Solid-State Fermentation.ACS Omega. 2023 Aug 23;8(36):32740-32751. doi: 10.1021/acsomega.3c03644. eCollection 2023 Sep 12. ACS Omega. 2023. PMID: 37720795 Free PMC article.
-
Application and characterization of crude fungal lipases used to degrade fat and oil wastes.Sci Rep. 2021 Oct 4;11(1):19670. doi: 10.1038/s41598-021-98927-4. Sci Rep. 2021. PMID: 34608188 Free PMC article.
References
-
- Bindiya P, Ramana T. Optimization of lipase production from an indigenously isolated marine Aspergillus sydowii of Bay of Bengal. J Biochem Tech. 2012;3:S203–S211.
-
- Castro-Ochoa LD, Rodriguez-Gomez C, Valerio-Alfaro G, Ros RO. Screening, purification and characterization of the thermoalkalophilic lipase produced by Bacillus thermoleovorans CCR11. Enzym Microb Technol. 2005;37:648–654. doi: 10.1016/j.enzmictec.2005.06.003. - DOI
-
- Chartrain M, Katz L, Marcin C, Thien M, Smith S, Fisher F, Goklen K, Salmon P, Brix T, Price K, Greasham R. Purification and characterization of a novel bioconverting lipase from Pseudomonas aeruginosa MB 5001. Enzym Microb Technol. 1993;15:575–580. doi: 10.1016/0141-0229(93)90019-X. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

