Xylanase production from Penicillium citrinum isolate HZN13 using response surface methodology and characterization of immobilized xylanase on glutaraldehyde-activated calcium-alginate beads
- PMID: 28330236
- PMCID: PMC4980835
- DOI: 10.1007/s13205-016-0484-9
Xylanase production from Penicillium citrinum isolate HZN13 using response surface methodology and characterization of immobilized xylanase on glutaraldehyde-activated calcium-alginate beads
Abstract
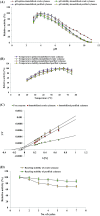
The present study reports the production of high-level cellulase-free xylanase from Penicillium citrinum isolate HZN13. The variability in xylanase titers was assessed under both solid-state (SSF) and submerged (SmF) fermentation. SSF was initially optimized with different agro-waste residues, among them sweet sorghum bagasse was found to be the best substrate that favored maximum xylanase production (9643 U/g). Plackett-Burman and response surface methodology employing central composite design were used to optimize the process parameters for the production of xylanase under SSF. A second-order quadratic model and response surface method revealed the optimum conditions for xylanase production (sweet sorghum bagasse 25 g/50 ml; ammonium sulphate 0.36 %; yeast extract 0.6 %; pH 4; temperature 40 °C) yielding 30,144 U/g. Analysis of variance (ANOVA) showed a high correlation coefficient (R 2 = 97.63 %). Glutaraldehyde-activated calcium-alginate-immobilized purified xylanase showed recycling stability (87 %) up to seven cycles. Immobilized purified xylanase showed enhanced thermo-stability in comparison to immobilized crude xylanase. Immobilization kinetics of crude and purified xylanase revealed an increase in K m (12.5 and 11.11 mg/ml) and V max (12,500 and 10,000 U/mg), respectively. Immobilized (crude) enzymatic hydrolysis of sweet sorghum bagasse released 8.1 g/g (48 h) of reducing sugars. Xylose and other oligosaccharides produced during hydrolysis were detected by High-Performance Liquid Chromatography. The biomass was characterized by Scanning Electron Microscopy, Energy Dispersive X-ray and Fourier Transformation Infrared Spectroscopy. However, this is one of the few reports on high-level cellulase-free xylanase from P. citrinum isolate using sweet sorghum bagasse.
Keywords: Enzymatic hydrolysis; Immobilization; Penicillium citrinum; Response surface methodology; Sweet sorghum bagasse; Xylanase.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Adapa PK, Tabil LG, Schoenau GJ, Canam T, Dumonceaux T. Quantitative analysis of lignocellulosic components of non-treated and steam exploded barley, canola, oat and wheat straw using fourier transform infrared spectroscopy. J Agri Sci Technol B. 2011;1:177–188.
-
- Adhyaru DN, NlS Bhatt, Modi HA. Optimization of upstream and downstream process parameters for cellulase-poor-thermo-solvent-stable xylanase production and extraction by Aspergillus tubingensis FDHN1. Bioresour Bioprocess. 2015;2:3. doi: 10.1186/s40643-014-0029-1. - DOI
-
- Aragon C, Mateo C, Ruiz-Matute A, Corzo N, Fernandez-Lorente G, Sevillano L, Díaz M, Monti R, Santamaría R, Guisan J. Production of xylo-oligosaccharides by immobilized-stabilized derivatives of endo-xylanase from Streptomyces halstedii. Process Biochem. 2013;48:478–483. doi: 10.1016/j.procbio.2013.01.010. - DOI
-
- Aragon C, Santos A, Ruiz-Matute A, Corzo N, Guisan J, Monti R, Mateo C. Continuous production of xylooligosaccharides in a packed bed reactor with immobilized–stabilized biocatalysts of xylanase from Aspergillus versicolor. J Mol Catal B Enzym. 2013;98:8–14. doi: 10.1016/j.molcatb.2013.09.017. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

