Calcium dysregulation, functional calpainopathy, and endoplasmic reticulum stress in sporadic inclusion body myositis
- PMID: 28330496
- PMCID: PMC5363023
- DOI: 10.1186/s40478-017-0427-7
Calcium dysregulation, functional calpainopathy, and endoplasmic reticulum stress in sporadic inclusion body myositis
Abstract
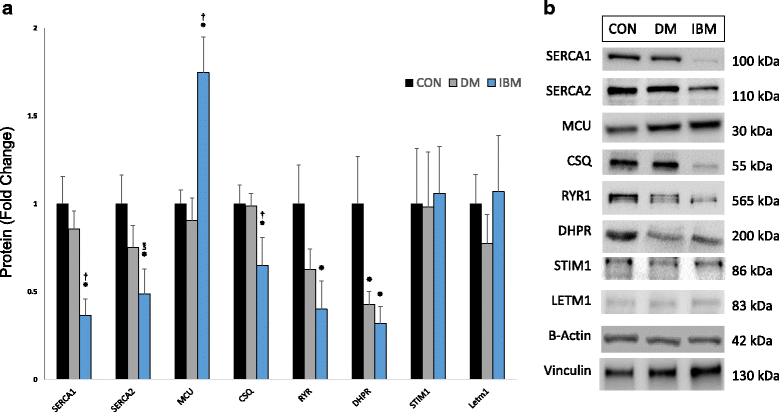
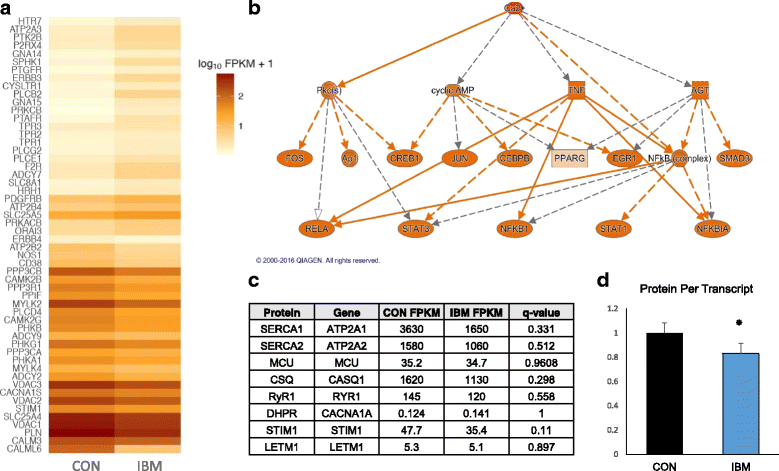
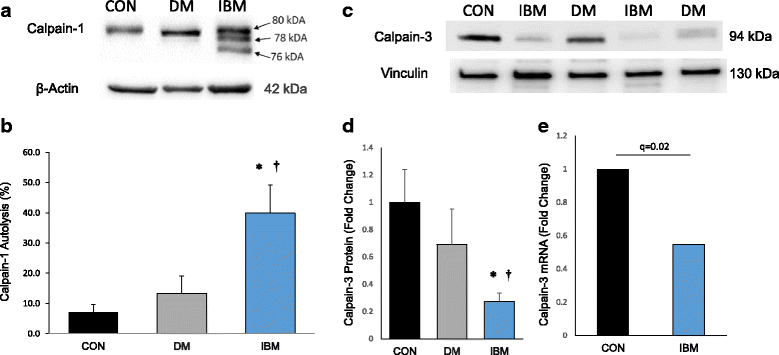
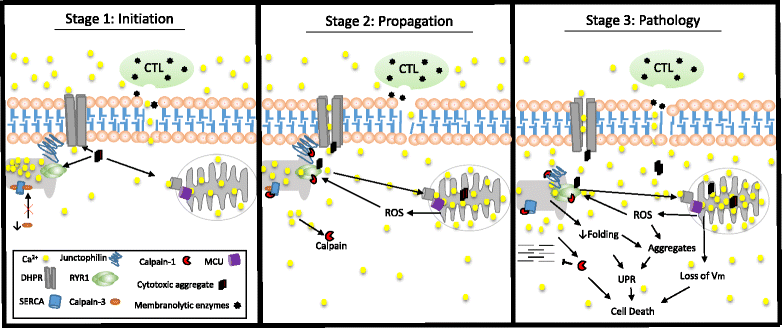
Sporadic inclusion body myositis (IBM) is the most common primary myopathy in the elderly, but its pathoetiology is still unclear. Perturbed myocellular calcium (Ca2+) homeostasis can exacerbate many of the factors proposed to mediate muscle degeneration in IBM, such as mitochondrial dysfunction, protein aggregation, and endoplasmic reticulum stress. Ca2+ dysregulation may plausibly be initiated in IBM by immune-mediated membrane damage and/or abnormally accumulating proteins, but no studies to date have investigated Ca2+ regulation in IBM patients. We first investigated protein expression via immunoblot in muscle biopsies from IBM, dermatomyositis, and non-myositis control patients, identifying several differentially expressed Ca2+-regulatory proteins in IBM. Next, we investigated the Ca2+-signaling transcriptome by RNA-seq, finding 54 of 183 (29.5%) genes from an unbiased list differentially expressed in IBM vs. controls. Using an established statistical approach to relate genes with causal transcription networks, Ca2+ abundance was considered a significant upstream regulator of observed whole-transcriptome changes. Post-hoc analyses of Ca2+-regulatory mRNA and protein data indicated a lower protein to transcript ratio in IBM vs. controls, which we hypothesized may relate to increased Ca2+-dependent proteolysis and decreased protein translation. Supporting this hypothesis, we observed robust (4-fold) elevation in the autolytic activation of a Ca2+-activated protease, calpain-1, as well as increased signaling for translational attenuation (eIF2a phosphorylation) downstream of the unfolded protein response. Finally, in IBM samples we observed mRNA and protein under-expression of calpain-3, the skeletal muscle-specific calpain, which broadly supports proper Ca2+ homeostasis. Together, these data provide novel insight into mechanisms by which intracellular Ca2+ regulation is perturbed in IBM and offer evidence of pathological downstream effects.
Keywords: Calcium; Calpain; Inclusion body; Muscular diseases; Myositis; Unfolded protein response.
Figures

References
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statists Soc. 1995;57:289–300.
-
- Benveniste O, Stenzel W, Hilton-Jones D, Sandri M, Boyer O, van Engelen BG. Amyloid deposits and inflammatory infiltrates in sporadic inclusion body myositis: the inflammatory egg comes before the degenerative chicken. Acta Neuropathol. 2015;129:611–624. doi: 10.1007/s00401-015-1384-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

