Stable and Potent Selenomab-Drug Conjugates
- PMID: 28330604
- PMCID: PMC5400723
- DOI: 10.1016/j.chembiol.2017.02.012
Stable and Potent Selenomab-Drug Conjugates
Abstract
Selenomabs are engineered monoclonal antibodies with one or more translationally incorporated selenocysteine residues. The unique reactivity of the selenol group of selenocysteine permits site-specific conjugation of drugs. Compared with other natural and unnatural amino acid and carbohydrate residues that have been used for the generation of site-specific antibody-drug conjugates, selenocysteine is particularly reactive, permitting fast, single-step, and efficient reactions under near physiological conditions. Using a tailored conjugation chemistry, we generated highly stable selenomab-drug conjugates and demonstrated their potency and selectivity in vitro and in vivo. These site-specific antibody-drug conjugates built on a selenocysteine interface revealed broad therapeutic utility in liquid and solid malignancy models.
Keywords: antibody-drug conjugates; cancer therapy; selenocysteine; site-specific conjugation.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

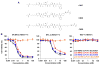
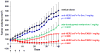


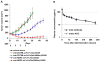
References
-
- Adair JR, Howard PW, Hartley JA, Williams DG, Chester KA. Antibody-drug conjugates - a perfect synergy. Expert Opin Biol Ther. 2012;12:1191–1206. - PubMed
-
- Alley SC, Benjamin DR, Jeffrey SC, Okeley NM, Meyer DL, Sanderson RJ, Senter PD. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug Chem. 2008;19:759–765. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

