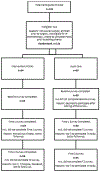
Rationale and study protocol for the Patient-Centered Outcome Aid (PCOA) randomized controlled trial: A personalized decision tool for newly diagnosed ovarian cancer patients
- PMID: 28330753
- PMCID: PMC6198815
- DOI: 10.1016/j.cct.2017.03.006
Rationale and study protocol for the Patient-Centered Outcome Aid (PCOA) randomized controlled trial: A personalized decision tool for newly diagnosed ovarian cancer patients
Figures
References
-
- American Cancer Society, Cancer Facts & Figures 2016, American Cancer Society, Atlanta, 2016.
-
- Alberts DS, et al., Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer, N. Engl. J. Med 335 (26) (1996) 1950–1955. - PubMed
-
- Armstrong DK, et al., Intraperitoneal cisplatin and paclitaxel in ovarian cancer, N. Engl. J. Med 354(1) (2006) 34–43. - PubMed
-
- Markman M, et al., Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group, J. Clin. Oncol 19 (4) (2001) 1001–1007. - PubMed
-
- Walker J, Brady M, DiSilvestro P, A phase III clinical trial of bevacizumab with IV versus IP chemotherapy in ovarian, fallopian tube and primary peritoneal carcinoma NCI-supplied agent (s): bevacizumab (NSC# 704865, IND# 7921) NCT01167712 a GOG/NRG trial (GOG 252), SGO Annual Meeting, 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical