Molecular Evidence for Differential Long-term Outcomes of Early Life Severe Acute Malnutrition
- PMID: 28330812
- PMCID: PMC5405153
- DOI: 10.1016/j.ebiom.2017.03.001
Molecular Evidence for Differential Long-term Outcomes of Early Life Severe Acute Malnutrition
Abstract
Background: Severe acute malnutrition (SAM) in infants may present as one of two distinct syndromic forms: non-edematous (marasmus), with severe wasting and no nutritional edema; or edematous (kwashiorkor) with moderately severe wasting. These differences may be related to developmental changes prior to the exposure to SAM and phenotypic changes appear to persist into adulthood with differences between the two groups. We examined whether the different response to SAM and subsequent trajectories may be explained by developmentally-induced epigenetic differences.
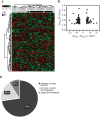
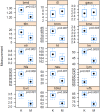
Methods: We extracted genomic DNA from muscle biopsies obtained from adult survivors of kwashiorkor (n=21) or marasmus (n=23) and compared epigenetic profiles (CpG methylation) between the two groups using the Infinium® 450K BeadChip array.
Findings: We found significant differences in methylation of CpG sites from 63 genes in skeletal muscle DNA. Gene ontology studies showed significant differential methylation of genes in immune, body composition, metabolic, musculoskeletal growth, neuronal function and cardiovascular pathways, pathways compatible with the differences in the pathophysiology of adult survivors of SAM.
Interpretation: These findings suggest persistent developmental influences on adult physiology in survivors of SAM. Since children who develop marasmus have lower birth weights and after rehabilitation have different intermediary metabolism, these studies provide further support for persistent developmentally-induced phenomena mediated by epigenetic processes affecting both the infant response to acute malnutrition and later life consequences.
Funding: Supported by a Grant from the Bill and Melinda Gates Foundation (Global Health OPP1066846), Grand Challenge "Discover New Ways to Achieve Healthy Growth."
Evidence before this study: Previous research has shown that infants who develop either kwashiorkor or marasmus in response to SAM differ in birth weight and subsequently have different metabolic patterns in both infancy and adulthood.
Added value of this study: This study demonstrates epigenetic differences in the skeletal muscle of adult survivors of marasmus versus kwashiorkor and these differences are in genes that may underlie the longer-term consequences.
Implications of all the available evidence: These data are compatible with the different clinical responses to SAM arising from developmentally-induced epigenetic changes laid down largely before birth and provide evidence for the predictive adaptive response model operating in human development.
Keywords: Birthweight; Epigenetic; Jamaica; Muscle; Severe acute malnutrition.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Edematous severe acute malnutrition is characterized by hypomethylation of DNA.Nat Commun. 2019 Dec 19;10(1):5791. doi: 10.1038/s41467-019-13433-6. Nat Commun. 2019. PMID: 31857576 Free PMC article.
-
Metabolomic Changes in Serum of Children with Different Clinical Diagnoses of Malnutrition.J Nutr. 2016 Dec;146(12):2436-2444. doi: 10.3945/jn.116.239145. Epub 2016 Nov 2. J Nutr. 2016. PMID: 27807038 Free PMC article.
-
The effect of wasting and stunting during severe acute malnutrition in infancy on insulin sensitivity and insulin clearance in adult life.J Dev Orig Health Dis. 2022 Dec;13(6):750-756. doi: 10.1017/S2040174422000034. Epub 2022 Mar 1. J Dev Orig Health Dis. 2022. PMID: 35229708
-
Difference between kwashiorkor and marasmus: Comparative meta-analysis of pathogenic characteristics and implications for treatment.Microb Pathog. 2021 Jan;150:104702. doi: 10.1016/j.micpath.2020.104702. Epub 2021 Jan 3. Microb Pathog. 2021. PMID: 33359074 Review.
-
Nutrition rehabilitation of children with severe acute malnutrition: Revisiting studies undertaken by the National Institute of Nutrition.Indian J Med Res. 2019 Aug;150(2):139-152. doi: 10.4103/ijmr.IJMR_1905_18. Indian J Med Res. 2019. PMID: 31670269 Free PMC article. Review.
Cited by
-
Severe malnutrition or famine exposure in childhood and cardiometabolic non-communicable disease later in life: a systematic review.BMJ Glob Health. 2021 Mar;6(3):e003161. doi: 10.1136/bmjgh-2020-003161. BMJ Glob Health. 2021. PMID: 33692144 Free PMC article.
-
Childhood severe acute malnutrition is associated with metabolomic changes in adulthood.JCI Insight. 2020 Dec 17;5(24):e141316. doi: 10.1172/jci.insight.141316. JCI Insight. 2020. PMID: 33201860 Free PMC article.
-
Edematous severe acute malnutrition is characterized by hypomethylation of DNA.Nat Commun. 2019 Dec 19;10(1):5791. doi: 10.1038/s41467-019-13433-6. Nat Commun. 2019. PMID: 31857576 Free PMC article.
-
Epigenetic Transgenerational Inheritance of Obesity Susceptibility.Trends Endocrinol Metab. 2020 Jul;31(7):478-494. doi: 10.1016/j.tem.2020.02.009. Epub 2020 Mar 24. Trends Endocrinol Metab. 2020. PMID: 32521235 Free PMC article. Review.
-
Evolutionary and developmental mismatches are consequences of adaptive developmental plasticity in humans and have implications for later disease risk.Philos Trans R Soc Lond B Biol Sci. 2019 Apr 15;374(1770):20180109. doi: 10.1098/rstb.2018.0109. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30966891 Free PMC article. Review.
References
-
- Al-Shahrour F., Diaz-Uriarte R., Dopazo J. FatiGO: a web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics. 2004;20(4):578–580. - PubMed
-
- Badaloo A., Reid M., Forrester T., Heird W.C., Jahoor F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am. J. Clin. Nutr. 2002;76(3):646–652. - PubMed
-
- Badaloo A.V., Forrester T., Reid M., Jahoor F. Lipid kinetic differences between children with kwashiorkor and those with marasmus. Am. J. Clin. Nutr. 2006;83(6):1283–1288. - PubMed
-
- Bateson P., Barker D., Clutton-Brock T. Developmental plasticity and human health. Nature. 2004;430:419–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

