A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice
- PMID: 28330852
- PMCID: PMC5471523
- DOI: 10.1096/fj.201700001R
A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice
Abstract
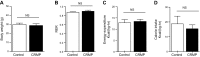
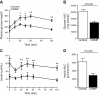
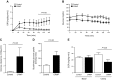
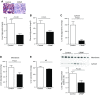
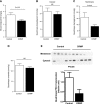
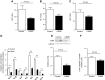
Lipodystrophy is a rare disorder characterized by complete or partial loss of adipose tissue. Patients with lipodystrophy exhibit hypertriglyceridemia, severe insulin resistance, type 2 diabetes, and nonalcoholic steatohepatitis (NASH). Efforts to ameliorate NASH in lipodystrophies with pharmacologic agents have met with limited success. We examined whether a controlled-release mitochondrial protonophore (CRMP) that produces mild liver-targeted mitochondrial uncoupling could decrease hypertriglyceridemia and reverse NASH and diabetes in a mouse model (fatless AZIP/F-1 mice) of severe lipodystrophy and diabetes. After 4 wk of oral CRMP (2 mg/kg body weight per day) or vehicle treatment, mice underwent hyperinsulinemic-euglycemic clamps combined with radiolabeled glucose to assess liver and muscle insulin responsiveness and tissue lipid measurements. CRMP treatment reversed hypertriglyceridemia and insulin resistance in liver and skeletal muscle. Reversal of insulin resistance could be attributed to reductions in diacylglycerol content and reduced PKC-ε and PKC-θ activity in liver and muscle respectively. CRMP treatment also reversed NASH as reflected by reductions in plasma aspartate aminotransferase and alanine aminotransferase concentrations; hepatic steatosis; and hepatic expression of IL-1α, -β, -2, -4, -6, -10, -12, CD69, and caspase 3 and attenuated activation of the IRE-1α branch of the unfolded protein response. Taken together, these results provide proof of concept for the development of liver-targeted mitochondrial uncoupling agents as a potential novel therapy for lipodystrophy-associated hypertriglyceridemia, NASH and diabetes.-Abulizi, A., Perry, R. J., Camporez, J. P. G., Jurczak, M. J., Petersen, K. F., Aspichueta, P., Shulman, G. I. A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice.
Keywords: NAFLD; NASH; insulin resistance; mitochondrial uncoupling; type 2 diabetes.
© FASEB.
Figures

References
-
- Garg A. (2000) Lipodystrophies. Am. J. Med. 108, 143–152 - PubMed
-
- Savage D. B. (2009) Mouse models of inherited lipodystrophy. Dis. Model. Mech. 2, 554–562 - PubMed
-
- Javor E. D., Ghany M. G., Cochran E. K., Oral E. A., DePaoli A. M., Premkumar A., Kleiner D. E., Gorden P. (2005) Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41, 753–760 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

