Influenza vaccines differentially regulate the interferon response in human dendritic cell subsets
- PMID: 28330867
- PMCID: PMC5484150
- DOI: 10.1126/scitranslmed.aaf9194
Influenza vaccines differentially regulate the interferon response in human dendritic cell subsets
Abstract
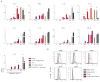
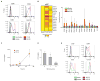
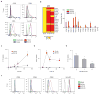
Human dendritic cells (DCs) play a fundamental role in the initiation of long-term adaptive immunity during vaccination against influenza. Understanding the early response of human DCs to vaccine exposure is thus essential to determine the nature and magnitude of maturation signals that have been shown to strongly correlate with vaccine effectiveness. In 2009, the H1N1 influenza epidemics fostered the commercialization of the nonadjuvanted monovalent H1N1 California vaccine (MIV-09) to complement the existing nonadjuvanted trivalent Fluzone 2009-2010 vaccine (TIV-09). In retrospective studies, MIV-09 displayed lower effectiveness than TIV-09. We show that TIV-09 induces monocyte-derived DCs (moDCs), blood conventional DCs (cDCs), and plasmacytoid DCs (pDCs) to express CD80, CD83, and CD86 and secrete cytokines. TIV-09 stimulated the secretion of type I interferons (IFNs) IFN-α and IFN-β and type III IFN interleukin-29 (IL-29) by moDC and cDC subsets. The vaccine also induced the production of IL-6, tumor necrosis factor, and the chemokines IFN-γ-inducible protein 10 (IP-10) and macrophage inflammatory protein-1β (MIP-1β). Conversely, MIV-09 did not induce the production of type I IFNs in moDCs and blood cDCs. Furthermore, it inhibited the TIV-09-induced secretion of type I IFNs by these DCs. However, both vaccines induced pDCs to secrete type I IFNs, indicating that different influenza vaccines activate distinct molecular signaling pathways in DC subsets. These results suggest that subtypes of nonadjuvanted influenza vaccines trigger immunity through different mechanisms and that the ability of a vaccine to induce an IFN response in DCs may offset the absence of adjuvant and increase vaccine efficacy.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures



References
-
- Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044. - PubMed
-
- Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. - PubMed
-
- Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

