Direct Detection of Rifampin and Isoniazid Resistance in Sputum Samples from Tuberculosis Patients by High-Resolution Melt Curve Analysis
- PMID: 28330890
- PMCID: PMC5442532
- DOI: 10.1128/JCM.02104-16
Direct Detection of Rifampin and Isoniazid Resistance in Sputum Samples from Tuberculosis Patients by High-Resolution Melt Curve Analysis
Abstract
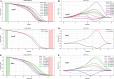
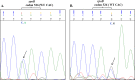
Drug-resistant tuberculosis (TB) is a major threat to TB control worldwide. Globally, only 40% of the 340,000 notified TB patients estimated to have multidrug-resistant-TB (MDR-TB) were detected in 2015. This study was carried out to evaluate the utility of high-resolution melt curve analysis (HRM) for the rapid and direct detection of MDR-TB in Mycobacterium tuberculosis in sputum samples. A reference plasmid library was first generated of the most frequently observed mutations in the resistance-determining regions of rpoB, katG, and an inhA promoter and used as positive controls in HRM. The assay was first validated in 25 MDR M. tuberculosis clinical isolates. The assay was evaluated on DNA isolated from 99 M. tuberculosis culture-positive sputum samples that included 84 smear-negative sputum samples, using DNA sequencing as gold standard. Mutants were discriminated from the wild type by comparing melting-curve patterns with those of control plasmids using HRM software. Rifampin (RIF) and isoniazid (INH) monoresistance were detected in 11 and 21 specimens, respectively, by HRM. Six samples were classified as MDR-TB by sequencing, one of which was missed by HRM. The HRM-RIF, INH-katG, and INH-inhA assays had 89% (95% confidence interval [CI], 52, 100%), 85% (95% CI, 62, 97%), and 100% (95% CI, 74, 100%) sensitivity, respectively, in smear-negative samples, while all assays had 100% sensitivity in smear-positive samples. All assays had 100% specificity. Concordance of 97% to 100% (κ value, 0.9 to 1) was noted between sequencing and HRM. Heteroresistance was observed in 5 of 99 samples by sequencing. In conclusion, the HRM assay was a cost-effective (Indian rupee [INR]400/US$6), rapid, and closed-tube method for the direct detection of MDR-TB in sputum, especially for direct smear-negative cases.
Keywords: DNA sequencing; high-resolution melt curve analysis; molecular diagnostics; multidrug resistance; sputum; tuberculosis.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- WHO. 2016. Global tuberculosis report. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?....
-
- UNITAID. 2013. Tuberculosis diagnostics technology and market landscape. UNITAID, Geneva, Switzerland: http://tbevidence.org/wp-content/uploads/2013/12/UNITAID-TB_Dx_Landscape....
-
- Nathavitharana RR, Hillemann D, Schumacher SG, Schlueter B, Ismail N, Omar SV, Sikhondze W, Havumaki J, Valli E, Boehme C, Denkinger CM. 2016. Multicenter noninferiority evaluation of Hain GenoType MTBDRplus version 2 and Nipro NTM+MDRTB line probe assays for detection of rifampin and isoniazid. J Clin Microbiol 54:1624–1630. - PMC - PubMed
-
- WHO. 2008. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB): policy statement. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/features_archive/policy_statement.pdf?ua=1.
-
- UNITAID. 2015. Tuberculosis diagnostics technology and market landscape, 4th ed UNITAID, Geneva, Switzerland: https://www.unitaid.eu/assets/Tuberculosis_diagnostics_technology_and_ma....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

