Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes
- PMID: 28330900
- PMCID: PMC5701572
- DOI: 10.4049/jimmunol.1601949
Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes
Abstract
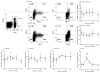
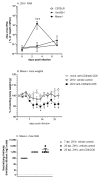
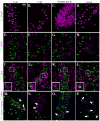
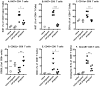
The recent association between Zika virus (ZIKV) and neurologic complications, including Guillain-Barré syndrome in adults and CNS abnormalities in fetuses, highlights the importance in understanding the immunological mechanisms controlling this emerging infection. Studies have indicated that ZIKV evades the human type I IFN response, suggesting a role for the adaptive immune response in resolving infection. However, the inability of ZIKV to antagonize the mouse IFN response renders the virus highly susceptible to circulating IFN in murine models. Thus, as we show in this article, although wild-type C57BL/6 mice mount cell-mediated and humoral adaptive immune responses to ZIKV, these responses were not required to prevent disease. However, when the type I IFN response of mice was suppressed, then the adaptive immune responses became critical. For example, when type I IFN signaling was blocked by Abs in Rag1-/- mice, the mice showed dramatic weight loss and ZIKV infection in the brain and testes. This phenotype was not observed in Ig-treated Rag1-/- mice or wild-type mice treated with anti-type I IFNR alone. Furthermore, we found that the CD8+ T cell responses of pregnant mice to ZIKV infection were diminished compared with nonpregnant mice. It is possible that diminished cell-mediated immunity during pregnancy could increase virus spread to the fetus. These results demonstrate an important role for the adaptive immune response in the control of ZIKV infection and imply that vaccination may prevent ZIKV-related disease, particularly when the type I IFN response is suppressed as it is in humans.
Figures





Similar articles
-
Using immunocompromised mice to identify mechanisms of Zika virus transmission and pathogenesis.Immunology. 2018 Apr;153(4):443-454. doi: 10.1111/imm.12883. Epub 2018 Jan 19. Immunology. 2018. PMID: 29266213 Free PMC article. Review.
-
Pregnancy Alters Innate and Adaptive Immune Responses to Zika Virus Infection in the Reproductive Tract.J Immunol. 2020 Dec 1;205(11):3107-3121. doi: 10.4049/jimmunol.2000882. Epub 2020 Oct 30. J Immunol. 2020. PMID: 33127823 Free PMC article.
-
A New In Vivo Model to Study Protective Immunity to Zika Virus Infection in Mice With Intact Type I Interferon Signaling.Front Immunol. 2018 Mar 22;9:593. doi: 10.3389/fimmu.2018.00593. eCollection 2018. Front Immunol. 2018. PMID: 29623081 Free PMC article.
-
CD8+ T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection.J Virol. 2017 Oct 27;91(22):e00900-17. doi: 10.1128/JVI.00900-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28835502 Free PMC article.
-
Zika Virus-A Reemerging Neurotropic Arbovirus Associated with Adverse Pregnancy Outcomes and Neuropathogenesis.Pathogens. 2024 Feb 15;13(2):177. doi: 10.3390/pathogens13020177. Pathogens. 2024. PMID: 38392915 Free PMC article. Review.
Cited by
-
Zika Virus Transmission Through Blood Tissue Barriers.Front Microbiol. 2019 Jul 4;10:1465. doi: 10.3389/fmicb.2019.01465. eCollection 2019. Front Microbiol. 2019. PMID: 31333605 Free PMC article. Review.
-
Microglia are involved in phagocytosis and extracellular digestion during Zika virus encephalitis in young adult immunodeficient mice.J Neuroinflammation. 2021 Aug 16;18(1):178. doi: 10.1186/s12974-021-02221-z. J Neuroinflammation. 2021. PMID: 34399779 Free PMC article.
-
Antiviral CD8 T cells induce Zika-virus-associated paralysis in mice.Nat Microbiol. 2018 Feb;3(2):141-147. doi: 10.1038/s41564-017-0060-z. Epub 2017 Nov 20. Nat Microbiol. 2018. PMID: 29158604 Free PMC article.
-
Using immunocompromised mice to identify mechanisms of Zika virus transmission and pathogenesis.Immunology. 2018 Apr;153(4):443-454. doi: 10.1111/imm.12883. Epub 2018 Jan 19. Immunology. 2018. PMID: 29266213 Free PMC article. Review.
-
Hide and Seek: The Interplay Between Zika Virus and the Host Immune Response.Front Immunol. 2021 Oct 21;12:750365. doi: 10.3389/fimmu.2021.750365. eCollection 2021. Front Immunol. 2021. PMID: 34745123 Free PMC article. Review.
References
-
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. - PubMed
-
- Heukelbach J, Werneck GL. Surveillance of Zika virus infection and microcephaly in Brazil. Lancet. 2016;388:846–847. - PubMed
-
- Magalhaes-Barbosa MC, Prata-Barbosa A, Robaina JR, Raymundo CE, Lima-Setta F, Cunha AJ. Trends of the microcephaly and Zika virus outbreak in Brazil, January–July 2016. Travel Med Infect Dis. 2016;14:458–463. - PubMed
-
- Meltzer E, Leshem E, Lustig Y, Gottesman G, Schwartz E. The Clinical Spectrum of Zika Virus in Returning Travelers. Am J Med. 2016;129:1126–1130. - PubMed
-
- Simpson DI. Zika Virus Infection in Man. Trans R Soc Trop Med Hyg. 1964;58:335–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

