Fundamental mechanisms of telomerase action in yeasts and mammals: understanding telomeres and telomerase in cancer cells
- PMID: 28330934
- PMCID: PMC5376709
- DOI: 10.1098/rsob.160338
Fundamental mechanisms of telomerase action in yeasts and mammals: understanding telomeres and telomerase in cancer cells
Abstract
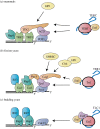
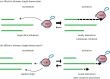
Aberrant activation of telomerase occurs in 85-90% of all cancers and underpins the ability of cancer cells to bypass their proliferative limit, rendering them immortal. The activity of telomerase is tightly controlled at multiple levels, from transcriptional regulation of the telomerase components to holoenzyme biogenesis and recruitment to the telomere, and finally activation and processivity. However, studies using cancer cell lines and other model systems have begun to reveal features of telomeres and telomerase that are unique to cancer. This review summarizes our current knowledge on the mechanisms of telomerase recruitment and activation using insights from studies in mammals and budding and fission yeasts. Finally, we discuss the differences in telomere homeostasis between normal cells and cancer cells, which may provide a foundation for telomere/telomerase targeted cancer treatments.
Keywords: Hayflick limit; S. pombe; replicative senescence; shelterin; t-stumps; telomere length homeostasis.
© 2017 The Authors.
Figures
References
-
- Szostak JW, Blackburn EH. 1982. Cloning yeast telomeres on linear plasmid vectors. Cell 29, 245–255. (doi:10.1016/0092-8674(82)90109-X) - DOI - PubMed
-
- Zakian VA. 1995. Telomeres: beginning to understand the end. Science 270, 1601–1607. (doi:10.1126/science.270.5242.1601) - DOI - PubMed
-
- Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, Shay JW, Ishioka S, Yamakido M. 1995. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 155, 3711–3715. - PubMed
-
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. 1996. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 18, 173–179. (doi:10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3) - DOI - PubMed
-
- Ramirez RD, Wright WE, Shay JW, Taylor RS. 1997. Telomerase activity concentrates in the mitotically active segments of human hair follicles. J. Invest. Dermatol. 108, 113–117. (doi:10.1111/1523-1747.ep12285654) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

