Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors
- PMID: 28331003
- PMCID: PMC5501742
- DOI: 10.1158/2159-8290.CD-16-1080
Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors
Erratum in
-
Correction: Phase I Dose-Escalation Study of Taselisib, an Oral PI3KInhibitor, in Patients with Advanced Solid Tumors.Cancer Discov. 2018 Nov;8(11):1491. doi: 10.1158/2159-8290.CD-18-1115. Cancer Discov. 2018. PMID: 30385526 No abstract available.
Abstract
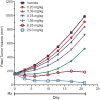
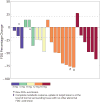
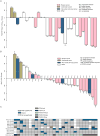
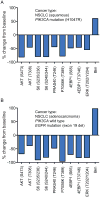
Taselisib is a potent and selective tumor growth inhibitor through PI3K pathway suppression. Thirty-four patients with locally advanced or metastatic solid tumors were treated (phase I study, modified 3+3 dose escalation; 5 cohorts; 3-16 mg taselisib once-daily capsule). Taselisib pharmacokinetics were dose-proportional; mean half-life was 40 hours. Frequent dose-dependent, treatment-related adverse events included diarrhea, hyperglycemia, decreased appetite, nausea, rash, stomatitis, and vomiting. At 12 and 16 mg dose levels, dose-limiting toxicities (DLT) were observed, with an accumulation of higher-grade adverse events after the cycle 1 DLT assessment window. Pharmacodynamic findings showed pathway inhibition at ≥3 mg in patient tumor samples, consistent with preclinical PIK3CA-mutant tumor xenograft models. Confirmed response rate was 36% for PIK3CA-mutant tumor patients with measurable disease [5/14: 4 breast cancer (3 patients at 12 mg); 1 non-small cell lung cancer], where responses started at 3 mg, and 0% in patients with tumors without known PIK3CA hotspot mutations (0/15).Significance: Preliminary data consistent with preclinical data indicate increased antitumor activity of taselisib in patients with PIK3CA-mutant tumors (in comparison with patients with tumors without known activating PIK3CA hotspot mutations) starting at the lowest dose tested of 3 mg, thereby supporting higher potency for taselisib against PIK3CA-mutant tumors. Cancer Discov; 7(7); 704-15. ©2017 AACR.See related commentary by Rodon and Tabernero, p. 666This article is highlighted in the In This Issue feature, p. 653.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
Comment in
-
Improving the Armamentarium of PI3K Inhibitors with Isoform-Selective Agents: A New Light in the Darkness.Cancer Discov. 2017 Jul;7(7):666-669. doi: 10.1158/2159-8290.CD-17-0500. Cancer Discov. 2017. PMID: 28684409
References
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. - PubMed
-
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. - PubMed
-
- Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

