Presynaptic and extrasynaptic regulation of posterior nucleus of thalamus
- PMID: 28331010
- PMCID: PMC5511867
- DOI: 10.1152/jn.00862.2016
Presynaptic and extrasynaptic regulation of posterior nucleus of thalamus
Abstract
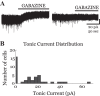
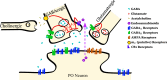
The posterior nucleus of thalamus (PO) is a higher-order nucleus involved in sensorimotor processing, including nociception. An important characteristic of PO is its wide range of activity profiles that vary across states of arousal, thought to underlie differences in somatosensory perception subject to attention and degree of consciousness. Furthermore, PO loses the ability to downregulate its activity level in some forms of chronic pain, suggesting that regulatory mechanisms underlying the normal modulation of PO activity may be pathologically altered. However, the mechanisms responsible for regulating such a wide dynamic range of activity are unknown. Here, we test a series of hypotheses regarding the function of several presynaptic receptors on both GABAergic and glutamatergic afferents targeting PO in mouse, using acute slice electrophysiology. We found that presynaptic GABAB receptors are present on both GABAergic and glutamatergic terminals in PO, but only those on GABAergic terminals are tonically active. We also found that release from GABAergic terminals, but not glutamatergic terminals, is suppressed by cholinergic activation and that a subpopulation of GABAergic terminals is regulated by cannabinoids. Finally, we discovered the presence of tonic currents mediated by extrasynaptic GABAA receptors in PO that are heterogeneously distributed across the nucleus. Thus we demonstrate that multiple regulatory mechanisms concurrently exist in PO, and we propose that regulation of inhibition, rather than excitation, is the more consequential mechanism by which PO activity can be regulated.NEW & NOTEWORTHY The posterior nucleus of thalamus (PO) is a key sensorimotor structure, whose activity is tightly regulated by inhibition from several nuclei. Maladaptive plasticity in this inhibition leads to severe pathologies, including chronic pain. We reveal here, for the first time in PO, multiple regulatory mechanisms that modulate synaptic transmission within PO. These findings may lead to targeted therapies for chronic pain and other disorders.
Keywords: GABA; glutamate; sensory processing; synaptic regulation.
Copyright © 2017 the American Physiological Society.
Figures





Similar articles
-
Pain After Spinal Cord Injury Is Associated With Abnormal Presynaptic Inhibition in the Posterior Nucleus of the Thalamus.J Pain. 2018 Jul;19(7):727.e1-727.e15. doi: 10.1016/j.jpain.2018.02.002. Epub 2018 Mar 2. J Pain. 2018. PMID: 29481977 Free PMC article.
-
Roles of GABAA and GABAB receptors in regulating thalamic activity by the zona incerta: a computational study.J Neurophysiol. 2014 Nov 15;112(10):2580-96. doi: 10.1152/jn.00282.2014. Epub 2014 Aug 20. J Neurophysiol. 2014. PMID: 25143541 Free PMC article.
-
Downregulation of tonic GABA currents following epileptogenic stimulation of rat hippocampal cultures.J Physiol. 2006 Dec 1;577(Pt 2):579-90. doi: 10.1113/jphysiol.2006.113134. Epub 2006 Sep 21. J Physiol. 2006. PMID: 16990405 Free PMC article.
-
GABA receptors and T-type Ca2+ channels crosstalk in thalamic networks.Neuropharmacology. 2018 Jul 1;136(Pt A):37-45. doi: 10.1016/j.neuropharm.2017.06.006. Epub 2017 Jun 7. Neuropharmacology. 2018. PMID: 28601398 Review.
-
Presynaptic glutamate receptors in nociception.Pharmacol Ther. 2023 Nov;251:108539. doi: 10.1016/j.pharmthera.2023.108539. Epub 2023 Sep 30. Pharmacol Ther. 2023. PMID: 37783347 Review.
Cited by
-
Role of Posterior Medial Thalamus in the Modulation of Striatal Circuitry and Choice Behavior.bioRxiv [Preprint]. 2024 Nov 8:2024.03.21.586152. doi: 10.1101/2024.03.21.586152. bioRxiv. 2024. Update in: Elife. 2025 May 13;13:RP98563. doi: 10.7554/eLife.98563. PMID: 38585753 Free PMC article. Updated. Preprint.
-
Presynaptic Inhibitory Effects of Acetylcholine in the Hippocampus: A 40-Year Evolution of a Serendipitous Finding.J Neurosci. 2021 May 26;41(21):4550-4555. doi: 10.1523/JNEUROSCI.3229-20.2021. Epub 2021 Apr 29. J Neurosci. 2021. PMID: 33926994 Free PMC article.
-
A glutamatergic innervation from medial area of secondary visual cortex to lateral posterior thalamic nucleus facilitates nociceptive and neuropathic pain.Commun Biol. 2025 Mar 11;8(1):416. doi: 10.1038/s42003-025-07874-7. Commun Biol. 2025. PMID: 40069548 Free PMC article.
-
Primary somatosensory cortex bidirectionally modulates sensory gain and nociceptive behavior in a layer-specific manner.Nat Commun. 2023 May 24;14(1):2999. doi: 10.1038/s41467-023-38798-7. Nat Commun. 2023. PMID: 37225702 Free PMC article.
-
Pain After Spinal Cord Injury Is Associated With Abnormal Presynaptic Inhibition in the Posterior Nucleus of the Thalamus.J Pain. 2018 Jul;19(7):727.e1-727.e15. doi: 10.1016/j.jpain.2018.02.002. Epub 2018 Mar 2. J Pain. 2018. PMID: 29481977 Free PMC article.
References
-
- Atwood HK, Malinow R, Manabe T, Poo M-m, Sakmann B, Silver PA. Central Synapses, Quantal Mechanisms and Plasticity (Human Frontier Workshop Reports, Workshop IV). Strasbourg, France: Human Frontier Science Program, 1998.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

