First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors
- PMID: 28331050
- PMCID: PMC7595575
- DOI: 10.1158/1078-0432.CCR-16-2658
First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors
Abstract
Purpose: ONC201 is a small-molecule selective antagonist of the G protein-coupled receptor DRD2 that is the founding member of the imipridone class of compounds. A first-in-human phase I study of ONC201 was conducted to determine its recommended phase II dose (RP2D).Experimental Design: This open-label study treated 10 patients during dose escalation with histologically confirmed advanced solid tumors. Patients received ONC201 orally once every 3 weeks, defined as one cycle, at doses from 125 to 625 mg using an accelerated titration design. An additional 18 patients were treated at the RP2D in an expansion phase to collect additional safety, pharmacokinetic, and pharmacodynamic information.Results: No grade >1 drug-related adverse events occurred, and the RP2D was defined as 625 mg. Pharmacokinetic analysis revealed a Cmax of 1.5 to 7.5 μg/mL (∼3.9-19.4 μmol/L), mean half-life of 11.3 hours, and mean AUC of 37.7 h·μg/L. Pharmacodynamic assays demonstrated induction of caspase-cleaved keratin 18 and prolactin as serum biomarkers of apoptosis and DRD2 antagonism, respectively. No objective responses by RECIST were achieved; however, radiographic regression of several individual metastatic lesions was observed along with prolonged stable disease (>9 cycles) in prostate and endometrial cancer patients.Conclusions: ONC201 is a selective DRD2 antagonist that is well tolerated, achieves micromolar plasma concentrations, and is biologically active in advanced cancer patients when orally administered at 625 mg every 3 weeks. Clin Cancer Res; 23(15); 4163-9. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
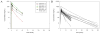
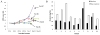

References
-
- Ishizawa J, Kojima K, Chachad D, Ruvolo P, Ruvolo V, Jacamo RO, Borthakur G, Mu H, Zeng Z, Tabe Y, Allen JE, Wang Z, Ma W, Lee HC, Orlowski R, Sarbassov DD, Lorenzi PL, Huang X, Neelapu SS, McDonnell T, Miranda RN, Wang M, Kantarjian H, Konopleva M, Davis RE, Andreeff M. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Science Signaling. 2016;9:ra17-ra17. - PMC - PubMed
-
- Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, Dolloff NG, Messaris E, Scata KA, Wang W, Zhou JY, Wu GS, El-Deiry WS. Dual Inactivation of Akt and ERK by TIC10 Signals Foxo3a Nuclear Translocation, TRAIL Gene Induction, and Potent Antitumor Effects. Science translational medicine. 2013;5:171ra117-171ra117. - PMC - PubMed
-
- Allen JE, Prabhu VV, Talekar M, van den Heuvel A, Lim B, Dicker DT, Fritz JL, Beck A, El-Deiry WS. Genetic and pharmacological screens converge in identifying FLIP, BCL2 and IAP proteins as key regulators of sensitivity to the TRAIL-inducing anti-cancer agent ONC201/TIC10. Cancer research. 2015 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

