Monkeypox Virus Host Factor Screen Using Haploid Cells Identifies Essential Role of GARP Complex in Extracellular Virus Formation
- PMID: 28331092
- PMCID: PMC5432867
- DOI: 10.1128/JVI.00011-17
Monkeypox Virus Host Factor Screen Using Haploid Cells Identifies Essential Role of GARP Complex in Extracellular Virus Formation
Abstract
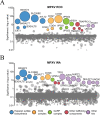
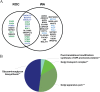
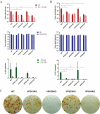
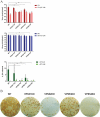
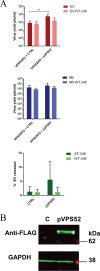
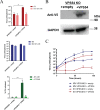
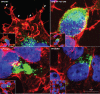
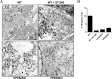
Monkeypox virus (MPXV) is a human pathogen that is a member of the Orthopoxvirus genus, which includes Vaccinia virus and Variola virus (the causative agent of smallpox). Human monkeypox is considered an emerging zoonotic infectious disease. To identify host factors required for MPXV infection, we performed a genome-wide insertional mutagenesis screen in human haploid cells. The screen revealed several candidate genes, including those involved in Golgi trafficking, glycosaminoglycan biosynthesis, and glycosylphosphatidylinositol (GPI)-anchor biosynthesis. We validated the role of a set of vacuolar protein sorting (VPS) genes during infection, VPS51 to VPS54 (VPS51-54), which comprise the Golgi-associated retrograde protein (GARP) complex. The GARP complex is a tethering complex involved in retrograde transport of endosomes to the trans-Golgi apparatus. Our data demonstrate that VPS52 and VPS54 were dispensable for mature virion (MV) production but were required for extracellular virus (EV) formation. For comparison, a known antiviral compound, ST-246, was used in our experiments, demonstrating that EV titers in VPS52 and VPS54 knockout (KO) cells were comparable to levels exhibited by ST-246-treated wild-type cells. Confocal microscopy was used to examine actin tail formation, one of the viral egress mechanisms for cell-to-cell dissemination, and revealed an absence of actin tails in VPS52KO- or VPS54KO-infected cells. Further evaluation of these cells by electron microscopy demonstrated a decrease in levels of wrapped viruses (WVs) compared to those seen with the wild-type control. Collectively, our data demonstrate the role of GARP complex genes in double-membrane wrapping of MVs necessary for EV formation, implicating the host endosomal trafficking pathway in orthopoxvirus infection.IMPORTANCE Human monkeypox is an emerging zoonotic infectious disease caused by Monkeypox virus (MPXV). Of the two MPXV clades, the Congo Basin strain is associated with severe disease, increased mortality, and increased human-to-human transmission relative to the West African strain. Monkeypox is endemic in regions of western and central Africa but was introduced into the United States in 2003 from the importation of infected animals. The threat of MPXV and other orthopoxviruses is increasing due to the absence of routine smallpox vaccination leading to a higher proportion of naive populations. In this study, we have identified and validated candidate genes that are required for MPXV infection, specifically, those associated with the Golgi-associated retrograde protein (GARP) complex. Identifying host targets required for infection that prevents extracellular virus formation such as the GARP complex or the retrograde pathway can provide a potential target for antiviral therapy.
Keywords: GARP complex; HAP1 screen; poxviruses; retrograde transport.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Damon IK. 2013. Poxviruses, p 2160–2184. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, Davidson W, Galloway R, Khristova ML, Reynolds MG, Zhao H, Carroll DS, Curns A, Formenty P, Esposito JJ, Regnery RL, Damon IK. 2005. A tale of two clades: monkeypox viruses. J Gen Virol 86:2661–2672. doi:10.1099/vir.0.81215-0. - DOI - PubMed
-
- Saijo M, Ami Y, Suzaki Y, Nagata N, Iwata N, Hasegawa H, Iizuka I, Shiota T, Sakai K, Ogata M, Fukushi S, Mizutani T, Sata T, Kurata T, Kurane I, Morikawa S. 2009. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J Gen Virol 90:2266–2271. doi:10.1099/vir.0.010207-0. - DOI - PubMed
-
- Learned LA, Reynolds MG, Wassa DW, Li Y, Olson VA, Karem K, Stempora LL, Braden ZH, Kline R, Likos A, Libama F, Moudzeo H, Bolanda JD, Tarangonia P, Boumandoki P, Formenty P, Harvey JM, Damon IK. 2005. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg 73:428–434. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

