Immune evasion of Borrelia miyamotoi: CbiA, a novel outer surface protein exhibiting complement binding and inactivating properties
- PMID: 28331202
- PMCID: PMC5428533
- DOI: 10.1038/s41598-017-00412-4
Immune evasion of Borrelia miyamotoi: CbiA, a novel outer surface protein exhibiting complement binding and inactivating properties
Erratum in
-
Author Correction: Immune evasion of Borrelia miyamotoi: CbiA, a novel outer surface protein exhibiting complement binding and inactivating properties.Sci Rep. 2019 May 2;9(1):7056. doi: 10.1038/s41598-019-42948-7. Sci Rep. 2019. PMID: 31043620 Free PMC article.
Abstract
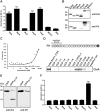
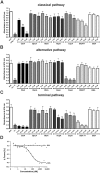
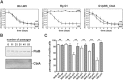
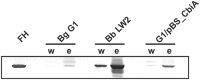
Borrelia (B.) miyamotoi, an emerging tick-borne relapsing fever spirochete, resists complement-mediated killing. To decipher the molecular principles of immune evasion, we sought to identify determinants contributing to complement resistance. Employing bioinformatics, we identified a gene encoding for a putative Factor H-binding protein, termed CbiA (complement binding and inhibitory protein A). Functional analyses revealed that CbiA interacted with complement regulator Factor H (FH), C3, C3b, C4b, C5, and C9. Upon binding to CbiA, FH retained its cofactor activity for Factor I-mediated inactivation of C3b. The Factor H-binding site within CbiA was mapped to domain 20 whereby the C-terminus of CbiA was involved in FH binding. Additionally, CbiA directly inhibited the activation of the classical pathway and the assembly of the terminal complement complex. Of importance, CbiA displayed inhibitory activity when ectopically produced in serum-sensitive B. garinii G1, rendering this surrogate strain resistant to human serum. In addition, long-term in vitro cultivation lead to an incremental loss of the cbiA gene accompanied by an increase in serum susceptibility. In conclusion, our data revealed a dual strategy of B. miyamotoi to efficiently evade complement via CbiA, which possesses complement binding and inhibitory activities.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Elucidating the Immune Evasion Mechanisms of Borrelia mayonii, the Causative Agent of Lyme Disease.Front Immunol. 2019 Nov 26;10:2722. doi: 10.3389/fimmu.2019.02722. eCollection 2019. Front Immunol. 2019. PMID: 31849943 Free PMC article.
-
Immune Evasion Strategies of Relapsing Fever Spirochetes.Front Immunol. 2020 Jul 23;11:1560. doi: 10.3389/fimmu.2020.01560. eCollection 2020. Front Immunol. 2020. PMID: 32793216 Free PMC article. Review.
-
The Complement Binding and Inhibitory Protein CbiA of Borrelia miyamotoi Degrades Extracellular Matrix Components by Interacting with Plasmin(ogen).Front Cell Infect Microbiol. 2018 Feb 2;8:23. doi: 10.3389/fcimb.2018.00023. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29456970 Free PMC article.
-
The relapsing fever spirochete Borrelia miyamotoi resists complement-mediated killing by human serum.Ticks Tick Borne Dis. 2014 Oct;5(6):898-901. doi: 10.1016/j.ttbdis.2014.07.011. Epub 2014 Jul 22. Ticks Tick Borne Dis. 2014. PMID: 25104575
-
Host Immune Evasion by Lyme and Relapsing Fever Borreliae: Findings to Lead Future Studies for Borrelia miyamotoi.Front Immunol. 2017 Jan 19;8:12. doi: 10.3389/fimmu.2017.00012. eCollection 2017. Front Immunol. 2017. PMID: 28154563 Free PMC article. Review.
Cited by
-
"Conformational dynamics of C1r inhibitor proteins from Lyme disease and relapsing fever spirochetes".bioRxiv [Preprint]. 2023 Mar 1:2023.03.01.530473. doi: 10.1101/2023.03.01.530473. bioRxiv. 2023. Update in: J Biol Chem. 2023 Aug;299(8):104972. doi: 10.1016/j.jbc.2023.104972. PMID: 36909632 Free PMC article. Updated. Preprint.
-
Pathogenesis of Relapsing Fever.Curr Issues Mol Biol. 2021;42:519-550. doi: 10.21775/cimb.042.519. Epub 2020 Dec 29. Curr Issues Mol Biol. 2021. PMID: 33372163 Free PMC article. Review.
-
Line Immunoblot Assay for Tick-Borne Relapsing Fever and Findings in Patient Sera from Australia, Ukraine and the USA.Healthcare (Basel). 2019 Oct 21;7(4):121. doi: 10.3390/healthcare7040121. Healthcare (Basel). 2019. PMID: 31640151 Free PMC article.
-
Elucidating the Immune Evasion Mechanisms of Borrelia mayonii, the Causative Agent of Lyme Disease.Front Immunol. 2019 Nov 26;10:2722. doi: 10.3389/fimmu.2019.02722. eCollection 2019. Front Immunol. 2019. PMID: 31849943 Free PMC article.
-
Immune Evasion Strategies of Relapsing Fever Spirochetes.Front Immunol. 2020 Jul 23;11:1560. doi: 10.3389/fimmu.2020.01560. eCollection 2020. Front Immunol. 2020. PMID: 32793216 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

