Traumatic brain injury results in acute rarefication of the vascular network
- PMID: 28331228
- PMCID: PMC5427893
- DOI: 10.1038/s41598-017-00161-4
Traumatic brain injury results in acute rarefication of the vascular network
Abstract
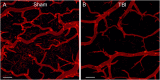
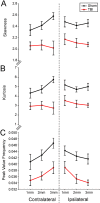
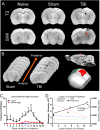
The role of the cerebrovascular network and its acute response to TBI is poorly defined and emerging evidence suggests that cerebrovascular reactivity is altered. We explored how cortical vessels are physically altered following TBI using a newly developed technique, vessel painting. We tested our hypothesis that a focal moderate TBI results in global decrements to structural aspects of the vasculature. Rats (naïve, sham-operated, TBI) underwent a moderate controlled cortical impact. Animals underwent vessel painting perfusion to label the entire cortex at 1 day post TBI followed by whole brain axial and coronal images using a wide-field fluorescence microscope. Cortical vessel network characteristics were analyzed for classical angiographic features (junctions, lengths) wherein we observed significant global (both hemispheres) reductions in vessel junctions and vessel lengths of 33% and 22%, respectively. Biological complexity can be quantified using fractal geometric features where we observed that fractal measures were also reduced significantly by 33%, 16% and 13% for kurtosis, peak value frequency and skewness, respectively. Acutely after TBI there is a reduction in vascular network and vascular complexity that are exacerbated at the lesion site and provide structural evidence for the bilateral hemodynamic alterations that have been reported in patients after TBI.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Ostergaard L, et al. Capillary transit time heterogeneity and flow-metabolism coupling after traumatic brain injury. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1585–1598. doi: 10.1038/jcbfm.2014.131. - DOI - PMC - PubMed
-
- Obenaus, A. Traumatic Brain Injury, In Encyclopedia of Mental Health, 2nd Edition (ed H., Friedman) Ch. 146, 329–240 (Elsevier, Inc., 2015).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

