Dentin sialoprotein facilitates dental mesenchymal cell differentiation and dentin formation
- PMID: 28331230
- PMCID: PMC5428264
- DOI: 10.1038/s41598-017-00339-w
Dentin sialoprotein facilitates dental mesenchymal cell differentiation and dentin formation
Abstract
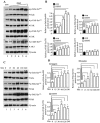
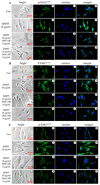
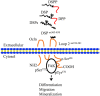
Dentin sialoprotein (DSP) is a dentin extracellular matrix protein. It is involved in dental mesenchymal cell lineages and dentin formation through regulation of its target gene expression. DSP mutations cause dentin genetic diseases. However, mechanisms of DSP in controlling dental mesenchymal cell differentiation are unknown. Using DSP as bait, we screened a protein library from mouse odontoblastic cells and found that DSP is a ligand and binds to cell surface receptor, occludin. Further study identified that the C-terminal DSP domainaa 363-458 interacts with the occludin extracellular loop 2aa 194-241. The C-terminal DSP domain induced phosphorylation of occludin Ser490 and focal adhesion kinase (FAK) Ser722 and Tyr576. Coexpression of DSP, occludin and FAK was detected in dental mesenchymal cells during tooth development. Occludin physically interacts with FAK, and occludin and FAK phosphorylation can be blocked by DSP and occludin antibodies. This DSP domain facilitates dental mesenchymal cell differentiation and mineralization. Furthermore, transplantation and pulp-capping procedures revealed that this DSP domain induces endogenous dental pulp mesenchymal cell proliferation, differentiation and migration, while stimulating blood vessel proliferation. This study elucidates the mechanism of DSP in dental mesenchymal lineages and implies that DSP may serve as a therapeutic agent for dentin-pulp complex regeneration in dental caries.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

