Restricted Axillary Staging in Clinically and Sonographically Node-Negative Early Invasive Breast Cancer (c/iT1-2) in the Context of Breast Conserving Therapy: First Results Following Commencement of the Intergroup-Sentinel-Mamma (INSEMA) Trial
- PMID: 28331237
- PMCID: PMC5357224
- DOI: 10.1055/s-0042-122853
Restricted Axillary Staging in Clinically and Sonographically Node-Negative Early Invasive Breast Cancer (c/iT1-2) in the Context of Breast Conserving Therapy: First Results Following Commencement of the Intergroup-Sentinel-Mamma (INSEMA) Trial
Abstract
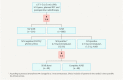
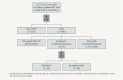
Axillary lymph node status remains an important prognostic factor in early breast cancer. It is regarded as an indicator for (neo)adjuvant systemic treatment and postoperative radiotherapy of the regional lymphatics. Commenced in September 2015, the INSEMA trial is investigating whether operative determination of nodal status as part of breast conserving therapy (BCT) for early stage breast cancer (c/iT1-2 c/iN0) can be avoided without reducing oncological safety. After inclusion of 1001 patients there was general acceptance of the complex study design by patients and study doctors so that recruitment for the first randomisation (axillary sentinel lymph node biopsy [SLNB]: yes or no) achieved predicted case numbers. The second randomisation however (SLNB alone versus complete axillary dissection when one or two macrometastases are present at SLNB) recruited fewer cases than expected for the following three reasons: a) the 13 % rate of one or two macrometastases after SLNB in the INSEMA trial collective was lower than expected; b) around 20 % of patients refused the second randomisation; c) there was delayed inclusion of the Austrian study centres, which only recruited for the second randomisation. Lack of knowledge of nodal status when SLNB is avoided represents a new challenge for the postoperative tumour board. In particular decisions on chemotherapy for luminal-like tumours and irradiation of the lymphatics (excluding axilla) must be guided by tumour biological parameters. The INSEMA trial does not provide answers to some important questions, e.g. it remains unclear whether patients without SLNB can be offered partial breast irradiation alone in low-risk situations and whether SLNB can also be avoided in patients with stage T1-2 tumours who have a mastectomy indication.
Der axilläre Nodalstatus wird beim frühen Mammakarzinom immer noch als wichtiger Prognosefaktor und Indikator für eine (neo-)adjuvante System- und postoperative Strahlentherapie der Lymphabflusswege (LAW) gesehen. Die im September 2015 gestartete INSEMA-Studie untersucht, ob beim frühen Mammakarzinom (c/iT1–2 c/iN0) auf die operative Bestimmung des Nodalstatus im Rahmen der brusterhaltenden Therapie (BET) verzichtet werden kann, ohne dass die onkologische Sicherheit beeinträchtigt wird. Nach Einschluss von 1001 Patientinnen war die Akzeptanz des komplexen Studiendesigns bei Patientinnen und Prüfärzten gegeben, sodass die Rekrutierung für die erste Randomisierung (axilläre Sentinel-Lymphknoten-Biopsie [SLNB]: ja oder nein) im Rahmen der Fallzahlprognose liegt. Die 2. Randomisierung (SLNB allein versus Komplettierung der Axilladissektion bei 1 oder 2 Makrometastasen in der SLNB) rekrutiert dagegen aus 3 Gründen weniger als erwartet: a) Der Nachweis von 1 bis 2 Makrometastasen nach der SLNB im INSEMA-Kollektiv ist mit 13 % geringer als erwartet; b) etwa 20 % der Patientinnen lehnten die 2. Randomisierung ab; c) der Einstieg der österreichischen Prüfzentren, die ausschließlich für die 2. Randomisierung rekrutieren, erfolgt zeitlich verzögert. Die Unkenntnis des Nodalstatus bei Verzicht auf die SLNB bringt eine neue Herausforderung für die postoperative Tumorkonferenz. Insbesondere die Indikation zur Chemotherapie bei Luminal-like-Tumoren und zur Bestrahlung der LAW (ohne Axilla) muss nun an den tumorbiologischen Parametern ausgerichtet werden. Einige wichtige Fragen können durch die INSEMA-Studie nicht beantwortet werden. Unklar bleibt beispielsweise, ob Patientinnen ohne SLNB eine alleinige Teilbrustbestrahlung in Low-Risk-Situationen angeboten werden kann bzw. ob auch bei Patientinnen mit einer Mastektomieindikation auf die SLNB im Stadium T1–2 verzichtet werden kann.
Keywords: INSEMA trial; breast cancer; nodal status; radiotherapy; sentinel lymph node biopsy.
Conflict of interest statement
Figures
References
-
- Gerber B, Heintze K, Stubert J. Axillary lymph node dissection in early-stage invasive breast cancer: is it still standard today? Breast Cancer Res Treat. 2011;128:613–624. - PubMed
-
- Gentilini O, Botteri E, Dadda P. Physical function of the upper limb after breast cancer surgery. Results from the SOUND (Sentinel node vs. Observation after axillary Ultra-souND) trial. Eur J Surg Oncol. 2016;42:685–689. - PubMed
-
- Lyman G H, Temin S, Edge S B. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–1383. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical