A Novel Role for Brain Natriuretic Peptide: Inhibition of IL-1β Secretion via Downregulation of NF-kB/Erk 1/2 and NALP3/ASC/Caspase-1 Activation in Human THP-1 Monocyte
- PMID: 28331244
- PMCID: PMC5346358
- DOI: 10.1155/2017/5858315
A Novel Role for Brain Natriuretic Peptide: Inhibition of IL-1β Secretion via Downregulation of NF-kB/Erk 1/2 and NALP3/ASC/Caspase-1 Activation in Human THP-1 Monocyte
Abstract
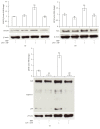
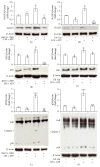
Interleukin-1β (IL-1β) is a pleiotropic cytokine and a crucial mediator of inflammatory and immune responses. IL-1β processing and release are tightly controlled by complex pathways such as NF-kB/ERK1/2, to produce pro-IL-1β, and NALP3/ASC/Caspase-1 inflammasome, to produce the active secreted protein. Dysregulation of both IL-1β and its related pathways is involved in inflammatory/autoimmune disorders and in a wide range of other diseases. Identifying molecules modulating their expression is a crucial need to develop new therapeutic agents. IL-1β is a strong regulator of Brain Natriuretic Peptide (BNP), a hormone involved in cardiovascular homeostasis by guanylyl cyclase Natriuretic Peptide Receptor (NPR-1). An emerging role of BNP in inflammation and immunity, although proposed, remains largely unexplored. Here, we newly demonstrated that, in human THP-1 monocytes, LPS/ATP-induced IL-1β secretion is strongly inhibited by BNP/NPR-1/cGMP axis at all the molecular mechanisms that tightly control its production and release, NF-kB, ERK 1/2, and all the elements of NALP3/ASC/Caspase-1 inflammasome cascade, and that NALP3 inflammasome inhibition is directly related to BNP deregulatory effect on NF-kB/ERK 1/2 activation. Our findings reveal a novel potent anti-inflammatory and immunomodulatory role for BNP and open new alleys of investigation for a possible employment of this endogenous agent in the treatment of inflammatory/immune-related and IL-1β/NF-kB/ERK1/2/NALP3/ASC/Caspase-1-associated diseases.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this study.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

