An open-label, two-period comparative study on pharmacokinetics and safety of a combined ethinylestradiol/gestodene transdermal contraceptive patch
- PMID: 28331292
- PMCID: PMC5354542
- DOI: 10.2147/DDDT.S131123
An open-label, two-period comparative study on pharmacokinetics and safety of a combined ethinylestradiol/gestodene transdermal contraceptive patch
Abstract
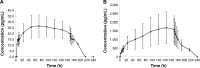
We investigated the pharmacokinetics and safety profiles of a newly developed combined ethinylestradiol (EE)/gestodene (GSD) transdermal contraceptive patch after a single-dose administration and compared with the market available tablet formulation in healthy adult subjects. An open-label, two-period comparative study was conducted in 12 healthy women volunteers. A single dose of the study combined EE/GE transdermal contraceptive patch and oral tablet (Milunet®) were administered. Blood samples at different time points after dose were collected, and concentrations were analyzed. A reliable, highly sensitive and accurate high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC/MS/MS) assay method was developed in this study to determine the plasma concentrations of EE and GSD. Compared to the tablet, the study patch had a significantly decreased maximum plasma concentration (Cmax), extended time to reach the Cmax and half-life, as well as increased clearance and apparent volume of distribution. The half-lives of EE and GSD of the patch were 3.3 and 2.2 times, respectively, than the half-life of the tablet. The areas under the plasma concentration-time curve (AUCs) of EE and GSD of the patch were 8.0 and 16.2 times, respectively, than the AUC of the tablet. No severe adverse event was observed during the whole study, and the general safety was acceptable. In conclusion, compared to the oral tablet Milunet, the study contraceptive patch was well tolerated and showed potent drug exposure, significant extended half-life and stable drug concentrations.
Keywords: ethinylestradiol/gestodene; pharmacokinetics; safety; transdermal contraceptive patch.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Daniels K, Daugherry J, Jones J. Current contraceptive status among women aged 15–44: United States, 2011–2013. NCHS Data Brief. 2014;(173):1–8. - PubMed
-
- Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995;51(5):283–288. - PubMed
-
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(suppl l):3–63. - PubMed
-
- Jung Hoffmann C, Kuhl H. Intra- and interindividual variations in contraceptive steroid levels during 12 treatment cycles: no relation to irregular bleedings. Contraception. 1990;42(4):423–438. - PubMed
-
- Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHSs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87(1):93–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

