Control of moderate-to-severe asthma with randomized ciclesonide doses of 160, 320 and 640 μg/day
- PMID: 28331346
- PMCID: PMC5349703
- DOI: 10.2147/JAA.S111712
Control of moderate-to-severe asthma with randomized ciclesonide doses of 160, 320 and 640 μg/day
Abstract
Background: The inhaled corticoteroid (ICS) ciclesonide (Cic), controls asthma symptoms in the majority of patients at the recommended dose of 160 µg/day. However, the relationship between the level of asthma control and increasing doses of Cic is unknown. This study investigated whether long-term treatment with higher doses of Cic would further improve asthma symptoms in patients with uncontrolled asthma despite ICS use.
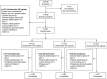
Patients and methods: In a double-blind, randomized, parallel-group study, 367 patients were allocated to one of three treatment arms (Cic 160, 320 and 640 µg/day). After a single-blind, 3-week baseline period with Cic 160 µg/day, eligible patients were randomized to receive 52 weeks of treatment with Cic 160, 320 or 640 µg/day (double-blind period) during which forced expiratory volume in 1 second (FEV1), exacerbations and Asthma Control Questionnaire (ACQ) scores were measured.
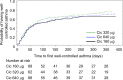
Results: Treatment with all the three doses was associated with significant improvements in ACQ scores, FEV1 and asthma symptoms (P<0.01). There were no statistically significant differences between the three doses. The results of the primary end point analysis showed a numerical improvement in the ACQ score with Cic 640 µg/day compared with Cic 160 µg/day (least square [LS] mean: -0.122; two-sided P-value: 0.30). Post hoc subgroup analyses showed that the improvement in the ACQ score with Cic 640 µg/day compared with Cic 160 µg/day was statistically significant in subjects who experience at least one exacerbation per year (LS mean: -0.586; 95% confidence interval: -1.110, -0.062, P=0.0285). Adverse events were low and consistent with the known safety profile of Cic.
Conclusion: In patients with persistent, uncontrolled asthma, increasing the Cic dose from 160 to 640 µg/day provided no clear additional effect. Patients who experience more than one exacerbation per year may benefit from higher doses; however, further studies are necessary to confirm this. All Cic doses were well tolerated.
Keywords: asthma control; dose-response.
Conflict of interest statement
Disclosure Dr Søren E Pedersen reports personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Sandoz. Dirkje S Postma reports grants for research (for the University of Groningen) from AstraZeneca, Chiesi, Genentech, GSK and Roche. Fees for consultancies were given to the University of Groningen by AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Takeda and TEVA. Niyati Prasad, Udo-Michael Goehring and Henrik Andersson were employees of Takeda Pharmaceuticals International GmbH during the time the study was conducted. The authors report no other conflicts of interest in this work.
Figures
References
-
- Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) [homepage on the Internet] 2015. [Accessed January 19, 2017]. Available from: http://www.ginasthma.org/
-
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. - PubMed
-
- Juniper EF, Bousquet J, Abetz L, Bateman ED; Committee GOAL. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. - PubMed
-
- Juniper EF, Svensson K, Mörk A-C, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. - PubMed
-
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–844. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

