Feasibility and Safety of Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis: A Retrospective Cohort Study
- PMID: 28331493
- PMCID: PMC5346367
- DOI: 10.1155/2017/6852749
Feasibility and Safety of Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis: A Retrospective Cohort Study
Abstract
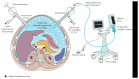
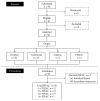
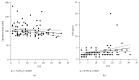
Background. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) has been introduced as a novel repeatable treatment for peritoneal carcinomatosis. The available evidence from the pioneer center suggests good tolerance and high response rates, but independent confirmation is needed. A single-center cohort was analyzed one year after implementation for feasibility and safety. Methods. PIPAC was started in January 2015, and every patient was entered into a prospective database. This retrospective analysis included all consecutive patients operated until April 2016 with emphasis on surgical feasibility and early postoperative outcomes. Results. Forty-two patients (M : F = 8 : 34, median age 66 (59-73) years) with 91 PIPAC procedures in total (4×: 1, 3×: 17, 2×: 12, and 1×: 12) were analyzed. Abdominal accessibility rate was 95% (42/44); laparoscopic access was not feasible in 2 patients with previous HIPEC. Median initial peritoneal carcinomatosis index (PCI) was 10 (IQR 5-17). Median operation time was 94 min (89-108) with no learning curve observed. One PIPAC application was postponed due to intraoperative intestinal lesion. Overall morbidity was 9% with 7 minor complications (Clavien I-II) and one PIPAC-unrelated postoperative mortality. Median postoperative hospital stay was 3 days (2-3). Conclusion. Repetitive PIPAC is feasible in most patients with refractory carcinomatosis of various origins. Intraoperative complications and postoperative morbidity rates were low. This encourages prospective studies assessing oncological efficacy.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures
Similar articles
-
Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis in Colorectal Cancer Patients: A Systematic Review of the Evidence.Cancers (Basel). 2024 Oct 30;16(21):3661. doi: 10.3390/cancers16213661. Cancers (Basel). 2024. PMID: 39518099 Free PMC article. Review.
-
Initial Single-center Experience of PIPAC in Patients With Unresectable Peritoneal Metastasis.Cir Esp (Engl Ed). 2021 May;99(5):354-360. doi: 10.1016/j.ciresp.2020.06.020. Epub 2020 Aug 3. Cir Esp (Engl Ed). 2021. PMID: 32762956 English, Spanish.
-
Impact of Pressurized Intraperitoneal Aerosol Chemotherapy on Quality of Life and Symptoms in Patients with Peritoneal Carcinomatosis: A Retrospective Cohort Study.Gastroenterol Res Pract. 2017;2017:4596176. doi: 10.1155/2017/4596176. Epub 2017 Feb 21. Gastroenterol Res Pract. 2017. PMID: 28316621 Free PMC article.
-
Pressurized IntraPeritoneal Aerosol Chemotherapy - Practical aspects.Eur J Surg Oncol. 2017 Jun;43(6):1102-1109. doi: 10.1016/j.ejso.2017.03.019. Epub 2017 Apr 8. Eur J Surg Oncol. 2017. PMID: 28431896
-
10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis.Cancers (Basel). 2023 Feb 9;15(4):1125. doi: 10.3390/cancers15041125. Cancers (Basel). 2023. PMID: 36831468 Free PMC article. Review.
Cited by
-
High-Risk Peritoneal Mesothelioma: Does Metronomic Chemotherapy Have a Role?Indian J Surg Oncol. 2023 Jun;14(Suppl 1):181-188. doi: 10.1007/s13193-022-01691-8. Epub 2023 Jan 5. Indian J Surg Oncol. 2023. PMID: 37359939 Free PMC article.
-
Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC-OX) in patients with colorectal peritoneal metastases-a systematic review.J Gastrointest Oncol. 2021 Apr;12(Suppl 1):S242-S258. doi: 10.21037/jgo-20-257. J Gastrointest Oncol. 2021. PMID: 33968441 Free PMC article. Review.
-
Repetitive electrostatic pressurised intraperitoneal aerosol chemotherapy (ePIPAC) with oxaliplatin as a palliative monotherapy for isolated unresectable colorectal peritoneal metastases: protocol of a Dutch, multicentre, open-label, single-arm, phase II study (CRC-PIPAC).BMJ Open. 2019 Jul 27;9(7):e030408. doi: 10.1136/bmjopen-2019-030408. BMJ Open. 2019. PMID: 31352425 Free PMC article.
-
Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis in Colorectal Cancer Patients: A Systematic Review of the Evidence.Cancers (Basel). 2024 Oct 30;16(21):3661. doi: 10.3390/cancers16213661. Cancers (Basel). 2024. PMID: 39518099 Free PMC article. Review.
-
Pressurised intraperitoneal aerosol chemotherapy (PIPAC): the first Australian experience.Pleura Peritoneum. 2025 Apr 9;10(1):25-31. doi: 10.1515/pp-2024-0028. eCollection 2025 Mar. Pleura Peritoneum. 2025. PMID: 40275878 Free PMC article.
References
-
- Klaver Y. L., Lemmens V. E., Creemers G. J., Rutten H. J., Nienhuijs S. W., de Hingh I. H. Population-based survival of patients with peritoneal carcinomatosis from colorectal origin in the era of increasing use of palliative chemotherapy. Annals of Oncology. 2011;22(10):2250–2256. doi: 10.1093/annonc/mdq762. - DOI - PubMed
-
- Franko J., Shi Q., Goldman C. D., et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. Journal of Clinical Oncology. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

