Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment
- PMID: 28331617
- PMCID: PMC5359946
- DOI: 10.1186/s40425-017-0230-9
Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment
Abstract
Chimeric antigen receptor (CAR) T-cell therapy represents a revolutionary treatment for haematological malignancies (i.e. B-ALL). However, the success of this type of treatment has not yet been achieved in solid tumors. One hypothesis is that the immunosuppressive nature of the tumor microenvironment (TME) influences and affects the efficacy of adoptive immunotherapy. Understanding the role of the TME and its interaction with CAR T-cells is crucial to improve the potency of adoptive immunotherapy. In this review, we discuss the strategies and potential combinatorial approaches recently developed in mouse models to enhance the efficacy of CAR T-cells, with particular emphasis on the translational potential of these approaches.
Keywords: Adoptive immunotherapy; Chimeric antigen receptor T-cells; Tumor microenvironment.
Figures
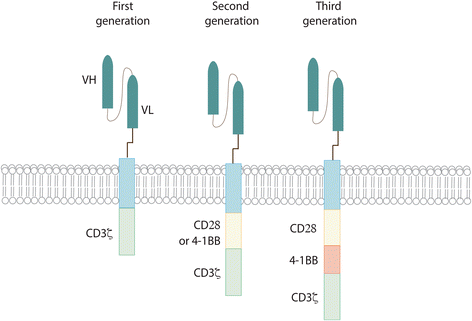
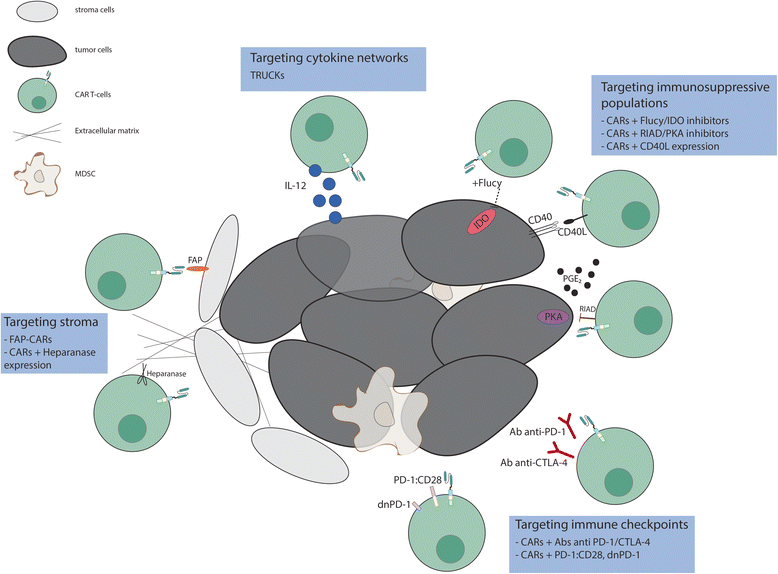
References
-
- Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Med Sci Transl. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. - DOI - PMC - PubMed
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. - DOI - PMC - PubMed
-
- Burns WR, Zheng Z, Rosenberg SA, Morgan RA. Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood. 2009;114:2888–2899. doi: 10.1182/blood-2009-01-199216. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
