IL-1β is an innate immune sensor of microbial proteolysis
- PMID: 28331908
- PMCID: PMC5358671
- DOI: 10.1126/sciimmunol.aah3539
IL-1β is an innate immune sensor of microbial proteolysis
Abstract
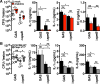
Interleukin-1β (IL-1β) is a key proinflammatory cytokine that drives antimicrobial immune responses. IL-1β is aberrantly activated in autoimmune diseases, and IL-1β inhibitors are used as therapeutic agents to treat patients with certain autoimmune disorders. Review of postmarketing surveillance of patients receiving IL-1β inhibitors found a disproportionate reporting of invasive infections by group A Streptococcus (GAS). IL-1β inhibition increased mouse susceptibility to GAS infection, but IL-1β was produced independent of canonical inflammasomes. Newly synthesized IL-1β has an amino-terminal prodomain that blocks signaling activity, which is usually proteolytically removed by caspase-1, a protease activated within the inflammasome structure. In place of host caspases, the secreted GAS cysteine protease SpeB generated mature IL-1β. During invasive infection, GAS isolates may acquire pathoadaptive mutations eliminating SpeB expression to evade detection by IL-1β. Pharmacological IL-1β inhibition alleviates this selective pressure, allowing invasive infection by nonpathoadapted GAS. Thus, IL-1β is a sensor that directly detects pathogen-associated proteolysis through an independent pathway operating in parallel with host inflammasomes. Because IL-1β function is maintained across species, yet cleavage by caspases does not appear to be, detection of microbial proteases may represent an ancestral system of innate immune regulation.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



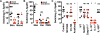
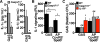

Comment in
-
Innate immunity: IL-1β activation under scrutiny.Nat Rev Immunol. 2016 Oct;16(10):594-5. doi: 10.1038/nri.2016.102. Epub 2016 Sep 12. Nat Rev Immunol. 2016. PMID: 27616587 No abstract available.
References
-
- Saavedra PH, Demon D, Van Gorp H, Lamkanfi M. Protective and detrimental roles of inflammasomes in disease. Semin Immunopathol. 2015;37:313–322. - PubMed
-
- Broderick L, De Nardo D, Franklin BS, Hoffman HM, Latz E. The inflammasome and autoinflammatory syndromes. Ann Rev Pathol. 2015;10:395–424. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

