Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions
- PMID: 28332093
- PMCID: PMC5486634
- DOI: 10.1007/s00401-017-1696-8
Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions
Abstract
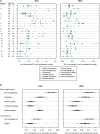
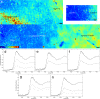
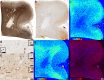
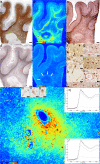
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) in which oligodendrocytes, the CNS cells that stain most robustly for iron and myelin are the targets of injury. Metals are essential for normal CNS functioning, and metal imbalances have been linked to demyelination and neurodegeneration. Using a multidisciplinary approach involving synchrotron techniques, iron histochemistry and immunohistochemistry, we compared the distribution and quantification of iron and zinc in MS lesions to the surrounding normal appearing and periplaque white matter, and assessed the involvement of these metals in MS lesion pathogenesis. We found that the distribution of iron and zinc is heterogeneous in MS plaques, and with few remarkable exceptions they do not accumulate in chronic MS lesions. We show that brain iron tends to decrease with increasing age and disease duration of MS patients; reactive astrocytes organized in large astrogliotic areas in a subset of smoldering and inactive plaques accumulate iron and safely store it in ferritin; a subset of smoldering lesions do not contain a rim of iron-loaded macrophages/microglia; and the iron content of shadow plaques varies with the stage of remyelination. Zinc in MS lesions was generally decreased, paralleling myelin loss. Iron accumulates concentrically in a subset of chronic inactive lesions suggesting that not all iron rims around MS lesions equate with smoldering plaques. Upon degeneration of iron-loaded microglia/macrophages, astrocytes may form an additional protective barrier that may prevent iron-induced oxidative damage.
Keywords: Astrocyte; Iron; Multiple sclerosis; Oligodendrocyte; Remyelination; Shadow plaque; Smoldering lesion; Synchrotron; Zinc.
Conflict of interest statement
Funding
This study was funded by the Canada Research Chairs Program (to BFP, GNG and IJP), Saskatchewan Health Research Foundation (to BFP), Biogen Idec (to BFP and CFL), and National Institutes of Health (to CFL). MT, RCA and SN were Fellows of the Canadian Institutes of Health Research Training grant in Health Research Using Synchrotron Techniques (CIHR-THRUST). MT was supported by a College of Medicine Graduate Scholarship, University of Saskatchewan and a Saskatchewan Innovation and Opportunity Scholarship, Government of Saskatchewan.
Conflict of interest
Dr. Popescu served as a speaker for Teva Innovation Canada, received honorarium for publishing in Continuum, received research support from the Saskatchewan Health Research Foundation and receives research support from the Canada Research Chairs program and Biogen Idec. Dr. Frischer was supported by the Austrian Science Fund (FWF Project J3508-B24) while conducting parts of this work. Dr. Metz reports grants from German Ministry for Education and Research (BMBF, ‘‘German Competence Network Multiple Sclerosis’’ (KKNMS), Pattern MS/NMO) and BiogenIdec as well as personal fees from BiogenIdec, Bayer Healthcare, TEVA, Serono and Novartis. Dr. Webb, Dr. Robinson, Dr. Adiele, Dr. Weigand, Mr. Fitz-Gibbon, Ms. Tham and Ms. Nehzati report no COI. Dr. Lassmann and Dr. Hametner receive research support from the Austrian Science Foundation and the Österreichische Nationalbank. Drs. George and Pickering receive research support from the Canada Research Chairs program, the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada and from Chevron USA and have received support from the Saskatchewan Health Research Foundation and from Grand Challenges Canada. Dr. Pickering is on the Board of Directors of the Canada Foundation for Innovation and receives additional research support from the Government of Canada. Dr. Brück receives research support from Teva Pharma, Biogen Idec, Novartis, and personal fees from Teva Pharma, Biogen Idec, Novartis, Merck-Serono, Bayer Vital, Genzyme. Dr Parisi receives publishing royalties for
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

