Development of Inhibitors Targeting Hypoxia-Inducible Factor 1 and 2 for Cancer Therapy
- PMID: 28332352
- PMCID: PMC5368132
- DOI: 10.3349/ymj.2017.58.3.489
Development of Inhibitors Targeting Hypoxia-Inducible Factor 1 and 2 for Cancer Therapy
Abstract
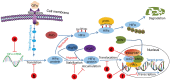
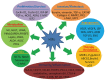
Hypoxia is frequently observed in solid tumors and also one of the major obstacles for effective cancer therapies. Cancer cells take advantage of their ability to adapt hypoxia to initiate a special transcriptional program that renders them more aggressive biological behaviors. Hypoxia-inducible factors (HIFs) are the key factors that control hypoxia-inducible pathways by regulating the expression of a vast array of genes involved in cancer progression and treatment resistance. HIFs, mainly HIF-1 and -2, have become potential targets for developing novel cancer therapeutics. This article reviews the updated information in tumor HIF pathways, particularly recent advances in the development of HIF inhibitors. These inhibitors interfere with mRNA expression, protein synthesis, protein degradation and dimerization, DNA binding and transcriptional activity of HIF-1 and -2, or both. Despite efforts in the past two decades, no agents directly inhibiting HIFs have been approved for treating cancer patients. By analyzing results of the published reports, we put the perspectives at the end of the article. The therapeutic efficacy of HIF inhibitors may be improved if more efforts are devoted on developing agents that are able to simultaneously target HIF-1 and -2, increasing the penetrating capacity of HIF inhibitors, and selecting suitable patient subpopulations for clinical trials.
Keywords: Hypoxia-inducible factor; anti-cancer drug; cancer; clinical trials.
© Copyright: Yonsei University College of Medicine 2017.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

