Pupillary responses in non-proliferative diabetic retinopathy
- PMID: 28332564
- PMCID: PMC5362954
- DOI: 10.1038/srep44987
Pupillary responses in non-proliferative diabetic retinopathy
Abstract
The goal of this study was to determine the extent of rod-, cone-, and melanopsin-mediated pupillary light reflex (PLR) abnormalities in diabetic patients who have non-proliferative diabetic retinopathy (NPDR). Fifty diabetic subjects who have different stages of NPDR and 25 age-equivalent, non-diabetic controls participated. PLRs were measured in response to full-field, brief-flash stimuli under conditions that target the rod, cone, and intrinsically-photosensitive (melanopsin) retinal ganglion cell pathways. Pupil responses were compared among the subjects groups using age-corrected linear mixed models. Compared to control, the mean baseline pupil diameters were significantly smaller for all patient groups in the dark (all p < 0.001) and for the moderate-severe NPDR group in the light (p = 0.003). Pairwise comparisons indicated: (1) the mean melanopsin-mediated PLR was significantly reduced in the mild and moderate-severe groups (both p < 0.001); (2) the mean cone-mediated PLR was reduced significantly in the moderate-severe group (p = 0.008); (3) no significant differences in the mean rod-mediated responses. The data indicate abnormalities in NPDR patients under conditions that separately assess pupil function driven by different photoreceptor classes. The results provide evidence for compromised neural function in these patients and provide a promising approach for quantifying their neural abnormalities.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
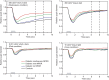


References
-
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services (2014).
-
- Wilkinson C. P. et al.. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 110, 1677–1682 (2003). - PubMed
-
- Lieth E., Gardner T. W., Barber A. J. & Antonetti D. A. & Penn State Retina Research, G. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 28, 3–8 (2000). - PubMed
-
- Adams A. J. & Bearse M. A. Jr. Retinal neuropathy precedes vasculopathy in diabetes: a function-based opportunity for early treatment intervention? Clin Experiment Ophthalmol. 95, 256–265 (2012). - PubMed
-
- Hreidarsson A. B. Pupil size in insulin-dependent diabetes. Relationship to duration, metabolic control, and long-term manifestations. Diabetes. 31, 442–448 (1982). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

