Immunogenicity of Candidate MERS-CoV DNA Vaccines Based on the Spike Protein
- PMID: 28332568
- PMCID: PMC5362948
- DOI: 10.1038/srep44875
Immunogenicity of Candidate MERS-CoV DNA Vaccines Based on the Spike Protein
Abstract
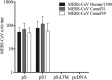
MERS-coronavirus is a novel zoonotic pathogen which spread rapidly to >25 countries since 2012. Its apparent endemicity and the wide spread of its reservoir host (dromedary camels) in the Arabian Peninsula highlight the ongoing public health threat of this virus. Therefore, development of effective prophylactic vaccine needs to be urgently explored given that there are no approved prophylactics or therapeutics for humans or animals to date. Different vaccine candidates have been investigated but serious safety concerns remain over protein or full-length spike (S) protein-based vaccines. Here, we investigated the immunogenicity of naked DNA vaccines expressing different fragments of MERS-CoV S protein in mice. We found that plasmids expressing full-length (pS) or S1-subunit (pS1) could induce significant levels of S1-specific antibodies (Abs) but with distinct IgG isotype patterns. Specifically, pS1 immunization elicited a balanced Th1/Th2 response and generally higher levels of all IgG isotypes compared to pS vaccination. Interestingly, only mice immunized with pS1 demonstrated significant S1-specific cellular immune response. Importantly, both constructs induced cross-neutralizing Abs against multiple strains of human and camel origins. These results indicate that vaccines expressing S1-subunit of the MERS-CoV S protein could represent a potential vaccine candidate without the possible safety concerns associated with full-length protein-based vaccines.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

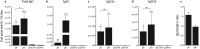

References
-
- Bermingham A. et al.. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro. Surveill. 17, 20290 (2012). - PubMed
-
- Pebody R. G. et al.. The United Kingdom public health response to an imported laboratory confirmed case of a novel coronavirus in September 2012. Euro. Surveill. 17, 20292 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

