Discovery of multi-target receptor tyrosine kinase inhibitors as novel anti-angiogenesis agents
- PMID: 28332573
- PMCID: PMC5362808
- DOI: 10.1038/srep45145
Discovery of multi-target receptor tyrosine kinase inhibitors as novel anti-angiogenesis agents
Abstract
Recently, we have identified a biphenyl-aryl urea incorporated with salicylaldoxime (BPS-7) as an anti-angiogenesis agent. Herein, we disclosed a series of novel anti-angiogenesis agents with BPS-7 as lead compound through combining diarylureas with N-pyridin-2-ylcyclopropane carboxamide. Several title compounds exhibited simultaneous inhibition effects against three pro-angiogenic RTKs (VEGFR-2, TIE-2 and EphB4). Some of them displayed potent anti-proliferative activity against human vascular endothelial cell (EA.hy926). In particular, two potent compounds (CDAU-1 and CDAU-2) could be considered as promising anti-angiogenesis agents with triplet inhibition profile. The biological evaluation and molecular docking results indicate that N-pyridin-2-ylcyclopropane carboxamide could serve as a hinge-binding group (HBG) for the discovery of multi-target anti-angiogenesis agents. CDAU-2 also exhibited promising anti-angiogenic potency in a tissue model for angiogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
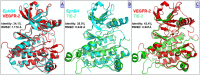






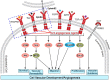
Similar articles
-
Discovery of novel anti-angiogenesis agents. Part 8: Diaryl thiourea bearing 1H-indazole-3-amine as multi-target RTKs inhibitors.Eur J Med Chem. 2017 Dec 1;141:373-385. doi: 10.1016/j.ejmech.2017.10.008. Epub 2017 Oct 6. Eur J Med Chem. 2017. PMID: 29032031
-
Discovery of novel anti-angiogenesis agents. Part 6: Multi-targeted RTK inhibitors.Eur J Med Chem. 2017 Feb 15;127:275-285. doi: 10.1016/j.ejmech.2016.12.059. Epub 2017 Jan 2. Eur J Med Chem. 2017. PMID: 28068599
-
Discovery of novel anti-angiogenesis agents. Part 7: Multitarget inhibitors of VEGFR-2, TIE-2 and EphB4.Eur J Med Chem. 2017 Dec 1;141:506-518. doi: 10.1016/j.ejmech.2017.10.030. Epub 2017 Oct 12. Eur J Med Chem. 2017. PMID: 29102175
-
New strategies in achieving antiangiogenic effect: Multiplex inhibitors suppressing compensatory activations of RTKs.Med Res Rev. 2018 Sep;38(5):1674-1705. doi: 10.1002/med.21517. Epub 2018 Jun 7. Med Res Rev. 2018. PMID: 29878411 Review.
-
Development of anti-angiogenic tyrosine kinases inhibitors: molecular structures and binding modes.Cancer Chemother Pharmacol. 2016 May;77(5):905-26. doi: 10.1007/s00280-016-2961-6. Epub 2016 Jan 18. Cancer Chemother Pharmacol. 2016. PMID: 26781310 Review.
Cited by
-
Research and development of N,N'-diarylureas as anti-tumor agents.RSC Med Chem. 2023 May 9;14(7):1209-1226. doi: 10.1039/d3md00053b. eCollection 2023 Jul 20. RSC Med Chem. 2023. PMID: 37484562 Free PMC article. Review.
-
Bioinformatics analysis reveals the potential target of rosiglitazone as an antiangiogenic agent for breast cancer therapy.BMC Genom Data. 2022 Sep 16;23(1):72. doi: 10.1186/s12863-022-01086-2. BMC Genom Data. 2022. PMID: 36114448 Free PMC article.
-
Synthetic and Naturally Occurring Heterocyclic Anticancer Compounds with Multiple Biological Targets.Molecules. 2021 Nov 25;26(23):7134. doi: 10.3390/molecules26237134. Molecules. 2021. PMID: 34885716 Free PMC article. Review.
-
Exploratory unsupervised machine learning of angiogenesis biomarkers in a phase II advanced cervical cancer trial of radiochemotherapy with or without neoadjuvant chemotherapy.Clinics (Sao Paulo). 2025 Jul 30;80:100723. doi: 10.1016/j.clinsp.2025.100723. Online ahead of print. Clinics (Sao Paulo). 2025. PMID: 40743651 Free PMC article.
-
KinaseFusionDB: an integrative knowledge of kinase fusion proteins in multi-scales.Brief Bioinform. 2025 May 1;26(3):bbaf259. doi: 10.1093/bib/bbaf259. Brief Bioinform. 2025. PMID: 40471992 Free PMC article.
References
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 438, 932–936 (2005). - PubMed
-
- Sato Y. Persistent vascular normalization as an alternative goal of anti-angiogenic cancer therapy. Cancer Sci. 102, 1253–1256 (2011). - PubMed
-
- Huang L. et al.. Development and strategies of VEGFR-2/KDR inhibitors. Future Med Chem. 4, 1839–1852 (2012). - PubMed
-
- Chioccioli M. et al.. Insights into the conformational switching mechanism of the human vascular endothelial growth factor receptor type 2 kinase domain. J Chem Inf Model. 52, 483–491 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

