Catalytic asymmetric radical aminoperfluoroalkylation and aminodifluoromethylation of alkenes to versatile enantioenriched-fluoroalkyl amines
- PMID: 28332576
- PMCID: PMC5376653
- DOI: 10.1038/ncomms14841
Catalytic asymmetric radical aminoperfluoroalkylation and aminodifluoromethylation of alkenes to versatile enantioenriched-fluoroalkyl amines
Abstract
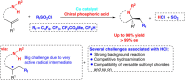
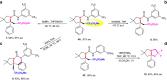
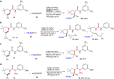
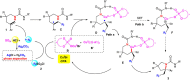
Although great success has been achieved in asymmetric fluoroalkylation reactions via nucleophilic or electrophilic processes, the development of asymmetric radical versions of this type of reactions remains a formidable challenge because of the involvement of highly reactive radical species. Here we report a catalytic asymmetric radical aminoperfluoroalkylation and aminodifluoromethylation of alkenes with commercially available fluoroalkylsulfonyl chlorides as the radical sources, providing a versatile platform to access four types of enantioenriched α-tertiary pyrrolidines bearing β-perfluorobutanyl, trifluoromethyl, difluoroacetyl and even difluoromethyl groups in excellent yields and with excellent enantioselectivity. The key to success is not only the introduction of the CuBr/chiral phosphoric acid dual-catalytic system but also the use of silver carbonate to suppress strong background and side hydroamination reactions caused by a stoichiometric amount of the in situ generated HCl. Broad substrate scope, excellent functional group tolerance and versatile functionalization of the products make this approach very practical and attractive.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Müller K., Faeh C. & Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007). - PubMed
-
- Purser S., Moore P. R., Swallow S. & Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008). - PubMed
-
- Wang J. et al.. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506 (2014). - PubMed
-
- Li Y. & Hu J. Facile synthesis of chiral α-difluoromethyl amines from N-(tert-butylsulfinyl)aldimines. Angew. Chem. Int. Ed. 44, 5882–5886 (2005). - PubMed
-
- Prakash G. K. S., Weber C., Chacko S. & Olah G. A. New electrophilic difluoromethylating reagent. Org. Lett. 9, 1863–1866 (2007). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

