Injections through skin colonized with Staphylococcus aureus biofilm introduce contamination despite standard antimicrobial preparation procedures
- PMID: 28332593
- PMCID: PMC5362901
- DOI: 10.1038/srep45070
Injections through skin colonized with Staphylococcus aureus biofilm introduce contamination despite standard antimicrobial preparation procedures
Abstract
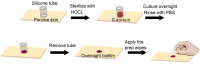
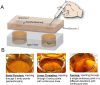
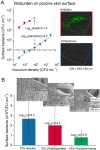
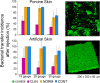
While surgical site preparation has been extensively studied, there is little information about resistance of skin microbiota in the biofilm form to antimicrobial decontamination, and there are no quantitative models to study how biofilm might be transferred into sterile tissue/implant materials during injections for joint spine and tendon, aspiration biopsies and dermal fillers (DF). In this work, we develop two in vitro models to simulate the process of skin preparation and DF injection using pig skin and SimSkin (silicone) materials, respectively. Using the pig skin model, we tested three of the most common skin preparation wipes (alcohol, chlorhexidine and povidone iodine) and found that during wiping they reduced the biofilm bacterial burden of S. aureus (CFU cm-2) by three logs with no statistically significant differences between wipes. Using the SimSkin model, we found that transfer of viable bacteria increased with needle diameter for 30G, 25G and 18G needles. Transfer incidence decreased as injection depth was increased from 1 mm to 3 mm. Serial puncture and linear threading injection styles had similar transfer incidence, whereas fanning significantly increased transfer incidence. The results show that contamination of DF during injection is a risk that can be reduced by modifying skin prep and injection practices.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
New in vitro model evaluating antiseptics' efficacy in biofilm-associated Staphylococcus aureus prosthetic vascular graft infection.J Med Microbiol. 2019 Mar;68(3):432-439. doi: 10.1099/jmm.0.000939. Epub 2019 Feb 8. J Med Microbiol. 2019. PMID: 30735113
-
Comparison of Decontamination Methods for Human Skin Grafts.J Burn Care Res. 2015 Nov-Dec;36(6):636-40. doi: 10.1097/BCR.0000000000000188. J Burn Care Res. 2015. PMID: 25383978
-
Methicillin-resistant Staphylococcus aureus colonization of infectious and non-infectious skin and soft tissue lesions in patients in Tehran.BMC Microbiol. 2021 Oct 18;21(1):282. doi: 10.1186/s12866-021-02340-w. BMC Microbiol. 2021. PMID: 34657594 Free PMC article.
-
Biofilm-based implant infections in orthopaedics.Adv Exp Med Biol. 2015;830:29-46. doi: 10.1007/978-3-319-11038-7_2. Adv Exp Med Biol. 2015. PMID: 25366219 Review.
-
Prevention and treatment of Staphylococcus aureus biofilms.Expert Rev Anti Infect Ther. 2015;13(12):1499-516. doi: 10.1586/14787210.2015.1100533. Epub 2015 Nov 13. Expert Rev Anti Infect Ther. 2015. PMID: 26646248 Free PMC article. Review.
Cited by
-
Removal of Staphylococcus aureus from skin using a combination antibiofilm approach.NPJ Biofilms Microbiomes. 2018 Aug 6;4:16. doi: 10.1038/s41522-018-0060-7. eCollection 2018. NPJ Biofilms Microbiomes. 2018. PMID: 30155267 Free PMC article.
-
Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo.PLoS One. 2018 Oct 10;13(10):e0205526. doi: 10.1371/journal.pone.0205526. eCollection 2018. PLoS One. 2018. PMID: 30304066 Free PMC article.
-
Process mapping strategies to prevent subcutaneous implantable cardioverter-defibrillator infections.J Cardiovasc Electrophysiol. 2022 Jul;33(7):1628-1635. doi: 10.1111/jce.15566. Epub 2022 Jun 9. J Cardiovasc Electrophysiol. 2022. PMID: 35662315 Free PMC article. Review.
-
Safety of intraarticular corticosteroid injection preceding hip and knee arthroplasty: a systematic review and meta-analysis amid resolving COVID-19 arthroplasty restrictions.J Hip Preserv Surg. 2021 Aug 24;8(3):215-224. doi: 10.1093/jhps/hnab064. eCollection 2021 Aug. J Hip Preserv Surg. 2021. PMID: 35578716 Free PMC article. Review.
-
An ex vivo model of medical device-mediated bacterial skin translocation.Sci Rep. 2021 Mar 11;11(1):5746. doi: 10.1038/s41598-021-84826-1. Sci Rep. 2021. PMID: 33707493 Free PMC article.
References
-
- Bangert C., Brunner P. M. & Stingl G. Immune functions of the skin. Clin. Dermatol. 29, 360–376 (2011). - PubMed
-
- Harrop J. S. et al.. Contributing Factors to Surgical Site Infections. J. Am. Acad. Orthop. Surg. 20, 94–101 (2012). - PubMed
-
- ASAPS. Cosmetic Surgery National Data Bank Statistics (The American Society for Aesthetic Plastic Surgery, 2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

