Janus-faced Acrolein prevents allergy but accelerates tumor growth by promoting immunoregulatory Foxp3+ cells: Mouse model for passive respiratory exposure
- PMID: 28332605
- PMCID: PMC5362909
- DOI: 10.1038/srep45067
Janus-faced Acrolein prevents allergy but accelerates tumor growth by promoting immunoregulatory Foxp3+ cells: Mouse model for passive respiratory exposure
Abstract
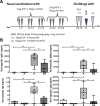
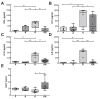
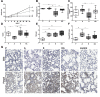
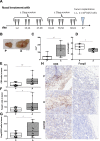
Acrolein, a highly reactive unsaturated aldehyde, is generated in large amounts during smoking and is best known for its genotoxic capacity. Here, we aimed to assess whether acrolein at concentrations relevant for smokers may also exert immunomodulatory effects that could be relevant in allergy or cancer. In a BALB/c allergy model repeated nasal exposure to acrolein abrogated allergen-specific antibody and cytokine formation, and led to a relative accumulation of regulatory T cells in the lungs. Only the acrolein-treated mice were protected from bronchial hyperreactivity as well as from anaphylactic reactions upon challenge with the specific allergen. Moreover, grafted D2F2 tumor cells grew faster and intratumoral Foxp3+ cell accumulation was observed in these mice compared to sham-treated controls. Results from reporter cell lines suggested that acrolein acts via the aryl-hydrocarbon receptor which could be inhibited by resveratrol and 3'-methoxy-4'-nitroflavone Acrolein- stimulation of human PBMCs increased Foxp3+ expression by T cells which could be antagonized by resveratrol. Our mouse and human data thus revealed that acrolein exerts systemic immunosuppression by promoting Foxp3+ regulatory cells. This provides a novel explanation why smokers have a lower allergy, but higher cancer risk.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Roy J., Pallepati P., Bettaieb A., Tanel A. & Averill-Bates D. A. Acrolein induces a cellular stress response and triggers mitochondrial apoptosis in A549 cells. Chem Biol Interact 181, 154–167 (2009). - PubMed
-
- Herrington J. S. & Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A(2015). - PubMed
-
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2, 372–377 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

